Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis
- PMID: 37629178
- PMCID: PMC10455111
- DOI: 10.3390/ijms241612998
Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis
Abstract
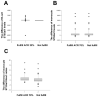
The enzymes α-2,6-sialyltransferase 1 (ST6Gal1), neuraminidase 1 (Neu1), α-2,3-sialyltransferase 1 (ST3Gal1), and neuraminidase 3 (Neu3) are known to affect immune cell function. However, it is not known whether the levels of these enzymes relate to remission definitions or differentiate American College of Rheumatology (ACR), European League Against Rheumatism (EULAR), and Simplified Disease Activity Index (SDAI) responses in patients with rheumatoid arthritis (RA). We measured the ST6Gal1, Neu1, ST3Gal1, and Neu3 levels of B cells and monocytes in RA patients and correlated the cells' enzyme levels/ratios with the improvement in the ACR, EULAR and SDAI responses and with the two remission definitions. The difference in the B-cell Neu1 levels differed between the ACR 70% improvement and non-improvement groups (p = 0.043), between the EULAR good major response (improvement) and non-good response groups (p = 0.014), and also between the SDAI 50% or 70% improvement and non-improvement groups (p = 0.001 and 0.018, respectively). The same held true when the RA patients were classified by positive rheumatoid factor or the use of biologics. The B-cell Neu1 levels significantly indicated 2005 modified American Rheumatism Association and 2011 ACR/EULAR remission definitions (area under the curve (AUC) = 0.674 with p = 0.001, and AUC = 0.682 with p < 0.001, respectively) in contrast to the CRP and ESR (all AUCs < 0.420). We suggest that B-cell Neu1 is superior for discriminating ACR, EULAR, and SDAI improvement and is good for predicting two kinds of remission definitions.
Keywords: B cells; Sialyltransferases; improvement criteria for RA; monocytes; neuraminidases; remission definitions for RA.
Conflict of interest statement
All authors declare no conflict of interest.
Figures




References
-
- Taylor W.J., Harrison A.A., Highton J., Chapma P., Stamp L., Dockerty J., McQueen F., Jones P.B.B., Ching D., Porter D., et al. Disease activity score 28-ESR bears a similar relationship to treatment decisions across different rheumatologists, but misclassification is to frequent to replace physician judgement. Rheumatology. 2008;47:514–518. doi: 10.1093/rheumatology/ken004. - DOI - PubMed
-
- Matsui T., Kuga Y., Kaneko A., Nishino J., Eto Y., Chiba N., Yasuda M., Saisho K., Shimada K., Tohma S. Disease activity score28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS 28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann. Rheum. Dis. 2007;66:1221–1226. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

