Unveiling the Link: Exploring Mitochondrial Dysfunction as a Probable Mechanism of Hepatic Damage in Post-Traumatic Stress Syndrome
- PMID: 37629192
- PMCID: PMC10455150
- DOI: 10.3390/ijms241613012
Unveiling the Link: Exploring Mitochondrial Dysfunction as a Probable Mechanism of Hepatic Damage in Post-Traumatic Stress Syndrome
Abstract
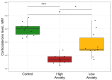
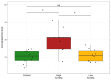
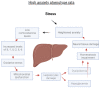
PTSD is associated with disturbed hepatic morphology and metabolism. Neuronal mitochondrial dysfunction is considered a subcellular determinant of PTSD, but a link between hepatic mitochondrial dysfunction and hepatic damage in PTSD has not been demonstrated. Thus, the effects of experimental PTSD on the livers of high anxiety (HA) and low anxiety (LA) rats were compared, and mitochondrial determinants underlying the difference in their hepatic damage were investigated. Rats were exposed to predator stress for 10 days. Then, 14 days post-stress, the rats were evaluated with an elevated plus maze and assigned to HA and LA groups according to their anxiety index. Experimental PTSD caused dystrophic changes in hepatocytes of HA rats and hepatocellular damage evident by increased plasma ALT and AST activities. Mitochondrial dysfunction was evident as a predominance of small-size mitochondria in HA rats, which was positively correlated with anxiety index, activities of plasma transaminases, hepatic lipids, and negatively correlated with hepatic glycogen. In contrast, LA rats had a predominance of medium-sized mitochondria. Thus, we show links between mitochondrial dysfunction, hepatic damage, and heightened anxiety in PTSD rats. These results will provide a foundation for future research on the role of hepatic dysfunction in PTSD pathogenesis.
Keywords: anxiety; cytokines; hepatocytes; inflammation; liver; mitochondria; oxidative stress; phenotypes; post-traumatic stress disorder; rats.
Conflict of interest statement
The authors declare no conflict of interest. The funding agencies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the manuscript.
Figures











References
-
- Smagin D., Kovalenko I., Galyamina A., Belozertseva I., Tamkovich N., Baranov K.O., Kudryavtseva N. Chronic lithium treatment affects anxious behaviors and the expression of serotonergic genes in midbrain raphe nuclei of defeated male mice. Biomedicines. 2021;22:1293. doi: 10.3390/biomedicines9101293. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

