Effectiveness and Safety of Xen Gel Stent in Glaucoma Surgery: A Systematic Review of the Literature
- PMID: 37629380
- PMCID: PMC10455777
- DOI: 10.3390/jcm12165339
Effectiveness and Safety of Xen Gel Stent in Glaucoma Surgery: A Systematic Review of the Literature
Abstract
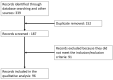
Although topical medical therapy and selective-laser-trabeculoplasty represent the treatments of choice to reduce intraocular pressure, many patients do not achieve adequate glaucoma control; therefore, they require further options and eventually surgery. Trabeculectomy is still considered the gold standard, but the surgical management of glaucoma has undergone continuous advances in recent years, XEN-gel-stent has been introduced as a safer and less traumatic means of lowering intraocular pressure (IOP) in patients with open-angle glaucoma (OAG). This study aimed to review the effectiveness and safety of clinical data on XEN-stent in OAG patients with a Synthesis-Without-Meta-analysis (SWiM) methodology. A total of 339 studies were identified following a literature search adhering to PRISMA guidelines and, after evaluation, 96 studies are discussed. XEN63 and XEN45 device data were collected both short and long term. In addition, this document has evaluated different aspects related to the XEN implant, including: its role compared to trabeculectomy; the impact of mitomycin-C dose on clinical outcomes; postoperative management of the device; and the identification of potential factors that might predict its clinical outcomes. Finally, current challenges and future perspectives of XEN stent, such as its use in fragile or high myopia patients, were discussed.
Keywords: XEN; glaucoma; microinvasive filtering-surgery; trabeculectomy.
Conflict of interest statement
This manuscript was funded by AbbVie S.r.l. AbbVie participated in writing, reviewing, and approving the publication. No honoraria or payments were made for authorship. C. Astarita and V. Vera are AbbVie employees and may own AbbVie stocks/options. B. Piergentili was a former AbbVie employee. She is now affiliated with Alnylam Italia Srl. She has nothing else to declare. A.M. Fea is consultant for Glaukos, EliosVision, Ivantis, Oculus, EyeD, iSTAR, Santen, Abbvie. S. Gandolfi research contracts and/or unrestricted research Grants from Ivantis, Glaukos, AERIE and Allergan/AbbVie, Advisory Board of Santen, Novartis, Baush and Lomb, Allergan/AbbVie, Fidia, Visufarma and AERIE, Speakers’ bureau of Allergan/AbbVie, Santen, Omikron, Visufarma, Baush and Lomb and Novartis. C. E. Traverso: support to the Department from Novartis, Bausch and Lomb, Alcon, Santen. Occasional travel support/speaker fee from Abbvie, Alcon, Santen, Omikron. R. Carassa and M. Figus have no conflicts of interest to declare.
Figures
References
-
- Heijl A., Leske M.C., Bengtsson B., Hyman L., Bengtsson B., Hussein M., Early Manifest Glaucoma Trial Group Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. - DOI - PubMed
-
- Lichter P.R., Musch D.C., Gillespie B.W., Guire K.E., Janz N.K., Wren P.A., CIGTS Study Group Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/S0161-6420(01)00873-9. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources