Comparison of MRI vs. [18F]FDG PET/CT for Treatment Response Evaluation of Primary Breast Cancer after Neoadjuvant Chemotherapy: Literature Review and Future Perspectives
- PMID: 37629397
- PMCID: PMC10455346
- DOI: 10.3390/jcm12165355
Comparison of MRI vs. [18F]FDG PET/CT for Treatment Response Evaluation of Primary Breast Cancer after Neoadjuvant Chemotherapy: Literature Review and Future Perspectives
Abstract
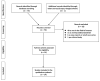
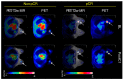
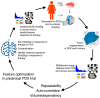
The purpose of this systematic review was to investigate the diagnostic accuracy of [18F]FDG PET/CT and breast MRI for primary breast cancer (BC) response assessment after neoadjuvant chemotherapy (NAC) and to evaluate future perspectives in this setting. We performed a critical review using three bibliographic databases (i.e., PubMed, Scopus, and Web of Science) for articles published up to the 6 June 2023, starting from 2012. The Quality Assessment of Diagnosis Accuracy Study (QUADAS-2) tool was adopted to evaluate the risk of bias. A total of 76 studies were identified and screened, while 14 articles were included in our systematic review after a full-text assessment. The total number of patients included was 842. Eight out of fourteen studies (57.1%) were prospective, while all except one study were conducted in a single center. In the majority of the included studies (71.4%), 3.0 Tesla (T) MRI scans were adopted. Three out of fourteen studies (21.4%) used both 1.5 and 3.0 T MRI and only two used 1.5 T. [18F]FDG was the radiotracer used in every study included. All patients accepted surgical treatment after NAC and each study used pathological complete response (pCR) as the reference standard. Some of the studies have demonstrated the superiority of [18F]FDG PET/CT, while others proved that MRI was superior to PET/CT. Recent studies indicate that PET/CT has a better specificity, while MRI has a superior sensitivity for assessing pCR in BC patients after NAC. The complementary value of the combined use of these modalities represents probably the most important tool to improve diagnostic performance in this setting. Overall, larger prospective studies, possibly randomized, are needed, hopefully evaluating PET/MR and allowing for new tools, such as radiomic parameters, to find a proper place in the setting of BC patients undergoing NAC.
Keywords: FDG; MRI; PET/CT; breast cancer; neoadjuvant chemotherapy; pathological complete response; response.
Conflict of interest statement
E.L. reports receiving grants from Fondazione AIRC (Associazione Italiana per la Ricerca sul Cancro) and the Italian Ministry of Health (Ministero della Salute), and faculty remuneration from ESMIT (European School of Multimodality Imaging & Therapy) and the MI&T congress. All remaining authors have declared no conflicts of interest.
Figures
References
-
- Korde L.A., Somerfield M.R., Carey L.A., Crews J.R., Denduluri N., Hwang E.S., Khan S.A., Loibl S., Morris E.A., Perez A., et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021;39:1485–1505. doi: 10.1200/JCO.20.03399. - DOI - PMC - PubMed
-
- Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., Zackrisson S., Senkus E. ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. - DOI - PubMed
-
- Laas E., Labrosse J., Hamy A.S., Benchimol G., de Croze D., Feron J.G., Coussy F., Balezeau T., Guerin J., Lae M., et al. Determination of breast cancer prognosis after neoadjuvant chemotherapy: Comparison of Residual Cancer Burden (RCB) and Neo-Bioscore. Br. J. Cancer. 2021;124:1421–1427. doi: 10.1038/s41416-020-01251-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

