SARS-CoV-2 Vaccination Response in Japanese Patients with Autoimmune Hepatitis: Results of Propensity Score-Matched Case-Control Study
- PMID: 37629453
- PMCID: PMC10455609
- DOI: 10.3390/jcm12165411
SARS-CoV-2 Vaccination Response in Japanese Patients with Autoimmune Hepatitis: Results of Propensity Score-Matched Case-Control Study
Abstract
Background/aims: Although the World Health Organization declared the end of the public health emergency of international concern focusing on COVID-19 in May 2023, this bothersome virus continues to mutate, and the possibility of the emergence of mutant strains with high infectivity and severe disease rates has not disappeared. Thus, medical evidence must be accumulated, which is indispensable for protecting both patients under immunosuppressive treatments and the healthy population. This study examined SARS-CoV-2 vaccination responses in Japanese patients with autoimmune hepatitis (AIH) compared with healthy controls.
Methods: This observational study registered 22 patients with histologically diagnosed AIH and 809 healthy controls in our hospital. Their Elecsys anti-SARS-CoV-2 spike antibody concentrations before and after vaccination were evaluated.
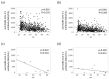
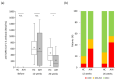
Results: In this study, 72.7% and 18.2% of patients with AIH received steroids and azathioprine, respectively. Significant negative correlations were found between age and anti-SARS-CoV-2 spike antibody concentration in both groups; however, no sex differences were found. Although anti-SARS-CoV-2 spike antibody concentration was drastically augmented after the second vaccination (p < 0.05) in the AIH group, these levels were significantly lower than those in the controls (p < 0.05). In the age- and sex-matched analysis, the population ratio with a minimum response (≤100 binding antibody units (BAU/mL) was higher among patients with AIH than among controls 26 weeks after the second vaccination (44% vs. 7%, p < 0.05).
Conclusions: The anti-SARS-CoV-2 spike antibody concentration in AIH patients was significantly lower than that in controls after the second vaccination. Continued and widespread vaccination, particularly for patients requiring medical immunomodulation, is recommended.
Keywords: COVID-19; SARS-CoV-2; autoimmune hepatitis; case–control studies; propensity score; vaccination.
Conflict of interest statement
The authors declare no conflict of interest for this article.
Figures


References
-
- Takahashi A., Moriya K., Ohira H., Arinaga-Hino T., Zeniya M., Torimura T., Abe M., Takaki A., Kang J.-H., Inui A., et al. Health-related quality of life in patients with autoimmune hepatitis: A questionnaire survey. PLoS ONE. 2018;13:e0204772. doi: 10.1371/journal.pone.0204772. - DOI - PMC - PubMed
-
- Lamba M., Ngu J.H., Stedman C.A.M. Trends in incidence of autoimmune liver diseases and increasing incidence of autoimmune hepatitis. Clin. Gastroenterol. Hepatol. 2021;19:573–579.e1. - PubMed
-
- Danielsson Borssén Å., Marschall H.U., Bergquist A., Rorsman F., Weiland O., Kechagias S., Nyhlin N., Verbaan H., Nilsson E., Werner M. Epidemiology and causes of death in a Swedish cohort of patients with autoimmune hepatitis. Scand. J. Gastroenterol. 2017;52:1022–1028. doi: 10.1080/00365521.2017.1335772. - DOI - PubMed
-
- Yoshizawa K., Matsumoto A., Ichijo T., Umemura T., Joshita S., Komatsu M., Tanaka N., Tanaka E., Ota M., Katsuyama Y. Long-term outcome of Japanese patients with type 1 autoimmune hepatitis. Hepatology. 2012;56:668–676. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

