Importance of Pharmacogenetics and Drug-Drug Interactions in a Kidney Transplanted Patient
- PMID: 37629484
- PMCID: PMC10455535
- DOI: 10.3390/life13081627
Importance of Pharmacogenetics and Drug-Drug Interactions in a Kidney Transplanted Patient
Abstract
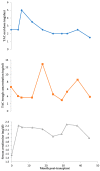
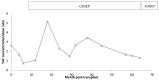
Tacrolimus (TAC) is a narrow-therapeutic-range immunosuppressant drug used after organ transplantation. A therapeutic failure is possible if drug levels are not within the therapeutic range after the first year of treatment. Pharmacogenetic variants and drug-drug interactions (DDIs) are involved. We describe a patient case of a young man (16 years old) with a renal transplant receiving therapy including TAC, mycophenolic acid (MFA), prednisone and omeprazole for prophylaxis of gastric and duodenal ulceration. The patient showed great fluctuation in TAC blood concentration/oral dose ratio, as well as pharmacotherapy adverse effects (AEs) and frequent diarrhea episodes. Additionally, decreased kidney function was found. A pharmacotherapeutic follow-up, including pharmacogenetic analysis, was carried out. The selection of the genes studied was based on the previous literature (CYP3A5, CYP3A4, POR, ABCB1, PXR and CYP2C19). A drug interaction with omeprazole was reported and the nephrologist switched to rabeprazole. A lower TAC concentration/dose ratio was achieved, and the patient's condition improved. In addition, the TTT haplotype of ATP Binding Cassette Subfamily B member 1 (ABCB1) and Pregnane X Receptor (PXR) gene variants seemed to affect TAC pharmacotherapy in the studied patient and could explain the occurrence of long-term adverse effects post-transplantation. These findings suggest that polymorphic variants and co-treatments must be considered in order to achieve the effectiveness of the immunosuppressive therapy with TAC, especially when polymedicated patients are involved. Moreover, pharmacogenetics could influence the drug concentration at the cellular level, both in lymphocyte and in renal tissue, and should be explored in future studies.
Keywords: drug–drug interaction; omeprazole; pharmacogenetics; polymorphisms; renal transplant; tacrolimus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sikma M.A., Van Maarseveen E.M., Hunault C.C., Moreno J.M., Van de Graaf E.A., Kirkels J.H., Verhaar M.C., Grutters J.C., Kesecioglu J., De Lange D.W., et al. Unbound Plasma, Total Plasma, and Whole-Blood Tacrolimus Pharmacokinetics Early After Thoracic Organ Transplantation. Clin. Pharmacokinet. 2020;59:771–780. doi: 10.1007/s40262-019-00854-1. - DOI - PMC - PubMed
-
- Bodnar-Broniarczyk M., Warzyszyńska K., Czerwińska K., Marszałek D., Dziewa N., Kosieradzki M., Pawiński T. Development and Validation of the New Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Unbound Tacrolimus in the Plasma Ultrafiltrate of Transplant Recipients. Pharmaceutics. 2022;14:632. doi: 10.3390/pharmaceutics14030632. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

