Tocilizumab Is Associated with Increased Risk of Fungal Infections among Critically Ill Patients with COVID-19 and Acute Renal Failure: An Observational Cohort Study
- PMID: 37629609
- PMCID: PMC10455962
- DOI: 10.3390/life13081752
Tocilizumab Is Associated with Increased Risk of Fungal Infections among Critically Ill Patients with COVID-19 and Acute Renal Failure: An Observational Cohort Study
Abstract
Research question: Does treatment with tocilizumab increase the risk of a fungal infection in critically ill patients with coronavirus-19?
Background: Numerous therapies have been evaluated as possible treatments for coronavirus-2019 caused by severe acute respiratory syndrome coronavirus-2. Tocilizumab is a humanized monoclonal antibody directed against the interleukin-6 receptor that has found a role as a therapy for patients with severe coronavirus-19 pneumonia. The immunomodulatory effects of tocilizumab may have the unintended consequence of predisposing recipients to secondary infections. We sought to assess the risk of invasive fungal disease and the therapeutic impact of tocilizumab on the hospital length of stay, duration of mechanical ventilation, and intensive-care-unit length of stay in critically ill patients with severe coronavirus-19 pneumonia.
Methods: Records of critically ill patients with coronavirus-2019 admitted from March to September 2020 at our institution were reviewed. The risk for fungal infections, intensive-care-unit length of stay, hospital length of stay, and duration of mechanical ventilation in those that received tocilizumab in addition to standard coronavirus-2019 treatments was assessed.
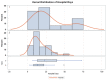
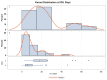
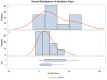
Results: Fifty-six critically ill patients treated with dexamethasone and remdesivir for coronavirus-2019 were included, of which 16 patients also received tocilizumab. The majority of the cohort was African American, Asian, or of other ethnic minorities (53.6%). Invasive fungal infections occurred in 10.7% of all patients, and infection rates were significantly higher in the tocilizumab group than in the control group (31.2% vs. 2.5%, risk difference [RD] = 28.8%, p < 0.01). The increased risk in the tocilizumab group was strongly associated with renal replacement therapy. There was a dose-response relationship between the risk of fungal infection and number of tocilizumab doses received, with 2.5% of infections occurring with zero doses, 20% with a single dose (RD = 17.5%), and 50% with two doses (RD = 47.5%) (trend test p < 0.001). In addition, ICU LOS (23.4 days vs. 9.0 days, p < 0.01), the duration of mechanical ventilation (18.9 vs. 3.5 days, p = 0.01), and hospital length of stay (LOS) (29.1 vs. 15.5, p < 0.01) were increased in patients that received tocilizumab.
Conclusions: Repurposed immunomodulator therapies, such as tocilizumab, are now recommended treatments for severe coronavirus-2019 pneumonia, but safety concerns remain. In this early pandemic cohort, the addition of tocilizumab to dexamethasone was associated with an increased risk of fungal infection in those that were critically ill and received renal replacement therapy. Tocilizumab use was also associated with increased ICU and hospital LOSs and duration of mechanical ventilation.
Keywords: COVID-19; fungal infection; fungal pneumonia; fungemia; renal replacement therapy; tocilizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Campbell L., Chen C., Bhagat S., Parker R., Ostor A. Risk of adverse events including serious infections in rheu-matoid arthritis patients treated with tocilizumab: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatology. 2011;50:552–562. doi: 10.1093/rheumatology/keq343. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

