Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation
- PMID: 37629800
- PMCID: PMC10456281
- DOI: 10.3390/ma16165509
Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation
Abstract
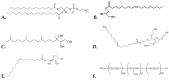
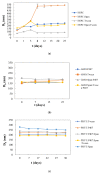
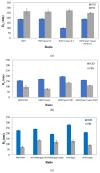
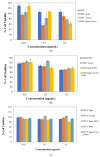
The aim of the present study is the development, physicochemical characterization, and in vitro cytotoxicity evaluation of both empty and quercetin-loaded HSPC (hydrogenated soy phosphatidylcholine) liposomes, GMO (glyceryl monooleate) liquid crystalline nanoparticles, and PHYT (phytantriol) liquid crystalline nanoparticles. Specifically, HSPC phospholipids were mixed with different non-ionic surfactant molecules (Tween 80 and/or Span 80) for liposomal formulations, whereas both GMO and PHYT lipids were mixed with Span 80 and Tween 80 as alternative stabilizers, as well as with Poloxamer P407 in different ratios for liquid crystalline formulations. Subsequently, their physicochemical properties, such as size, size distribution, and ζ-potential were assessed by the dynamic and electrophoretic light scattering (DLS/ELS) techniques in both aqueous and biological medium with serum proteins. The in vitro biological evaluation of the empty nanosystems was performed by using the MTT cell viability and proliferation assay. Finally, the entrapment efficiency of quercetin was calculated and the differences between the two different categories of lipidic nanoparticles were highlighted. According to the results, the incorporation of the non-ionic surfactants yields a successful stabilization and physicochemical stability of both liposomal and liquid crystalline nanoparticles. Moreover, in combination with an appropriate biosafety in vitro profile, increased encapsulation efficiency of quercetin was achieved. Overall, the addition of surfactants improved the nanosystem's stealth properties. In conclusion, the results indicate that the physicochemical properties were strictly affected by the formulation parameters, such as the type of surfactant.
Keywords: drug delivery nanosystems; lipid nanoparticles (LNPs); liposomes; lyotropic liquid crystals; quercetin; stealth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wadhwa K., Kadian V., Puri V., Bhardwaj B.Y., Sharma A., Pahwa R., Rao R., Gupta M., Singh I. New Insights into Quercetin Nanoformulations for Topical Delivery. Phytomed. Plus. 2022;2:100257. doi: 10.1016/j.phyplu.2022.100257. - DOI
LinkOut - more resources
Full Text Sources

