Recent Progress in Photonic Upconversion Materials for Organic Lanthanide Complexes
- PMID: 37629933
- PMCID: PMC10456671
- DOI: 10.3390/ma16165642
Recent Progress in Photonic Upconversion Materials for Organic Lanthanide Complexes
Abstract
Organic lanthanide complexes have garnered significant attention in various fields due to their intriguing energy transfer mechanism, enabling the upconversion (UC) of two or more low-energy photons into high-energy photons. In comparison to lanthanide-doped inorganic nanoparticles, organic UC complexes hold great promise for biological delivery applications due to their advantageous properties of controllable size and composition. This review aims to provide a summary of the fundamental concept and recent developments of organic lanthanide-based UC materials based on different mechanisms. Furthermore, we also detail recent applications in the fields of bioimaging and solar cells. The developments and forthcoming challenges in organic lanthanide-based UC offer readers valuable insights and opportunities to engage in further research endeavors.
Keywords: activator; mechanism; organic lanthanide complexes; sensitizer; upconversion luminescence.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Figures
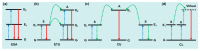
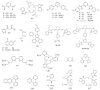


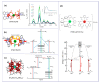
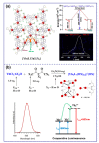

References
-
- Zheng X., Kankala R.K., Liu C.-G., Wang S.-B., Chen A.-Z., Zhang Y. Lanthanides-doped near-infrared active upconversion nanocrystals: Upconversion mechanisms and synthesis. Coord. Chem. Rev. 2021;438:213870–213887. doi: 10.1016/j.ccr.2021.213870. - DOI
-
- Healy C., Hermanspahn L., Kruger P.E. Photon upconversion in self-assembled materials. Coord. Chem. Rev. 2021;432:213756–213768. doi: 10.1016/j.ccr.2020.213756. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

