Silicon Carbide-Based DNA Sensing Technologies
- PMID: 37630093
- PMCID: PMC10456662
- DOI: 10.3390/mi14081557
Silicon Carbide-Based DNA Sensing Technologies
Abstract
DNA sensing is critical in various applications such as the early diagnosis of diseases and the investigation of forensic evidence, food processing, agriculture, environmental protection, etc. As a wide-bandgap semiconductor with excellent chemical, physical, electrical, and biocompatible properties, silicon carbide (SiC) is a promising material for DNA sensors. In recent years, a variety of SiC-based DNA-sensing technologies have been reported, such as nanoparticles and quantum dots, nanowires, nanopillars, and nanowire-based field-effect-transistors, etc. This article aims to provide a review of SiC-based DNA sensing technologies, their functions, and testing results.
Keywords: DNA; SiC; chemiresistor; label-free; sensitivity; sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



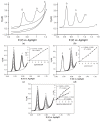



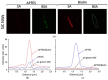

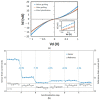
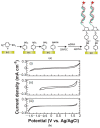
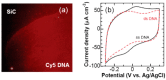
References
-
- Bano E., Fradetal L., Stambouli V., Attolini G. DNA Detection Using SiC Nanowire Based Transistor; Proceedings of the Silicon Carbide and Related Materials 2015; Giardini Naxos, Italy. 4–9 October 2015; Stafa-Zurich, Switzerland: Trans Tech Publications Ltd.; 2016. pp. 1006–1009.
-
- Frewin C.L., Nezafati M., Noble K., Saddow S.E. Chapter 2—Cytotoxicity of 3C–SiC Investigated through Strict Adherence to ISO 10993. In: Saddow S.E., editor. Silicon Carbide Biotechnology. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2016. pp. 27–61.
-
- Chen K.-I., Li B.-R., Chen Y.-T. Silicon Nanowire Field-Effect Transistor-Based Biosensors for Biomedical Diagnosis and Cellular Recording Investigation. Nano Today. 2011;6:131–154. doi: 10.1016/j.nantod.2011.02.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

