Simultaneous Hydrostatic and Compressive Loading System for Mimicking the Mechanical Environment of Living Cartilage Tissue
- PMID: 37630168
- PMCID: PMC10456493
- DOI: 10.3390/mi14081632
Simultaneous Hydrostatic and Compressive Loading System for Mimicking the Mechanical Environment of Living Cartilage Tissue
Abstract
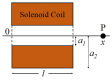
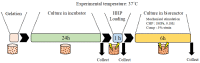
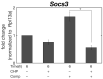
In vivo, articular cartilage tissue is surrounded by a cartilage membrane, and hydrostatic pressure (HP) and compressive strain increase simultaneously with the compressive stress. However, it has been impossible to investigate the effects of simultaneous loading in vitro. In this study, a bioreactor capable of applying compressive stress under HP was developed to reproduce ex vivo the same physical loading environment found in cartilage. First, a HP stimulation unit was constructed to apply a cyclic HP pressure-resistant chamber by controlling a pump and valve. A compression-loading mechanism that can apply compressive stress using an electromagnetic force was implemented in the chamber. The synchronization between the compression and HP units was evaluated, and the stimulation parameters were quantitatively evaluated. Physiological HP and compressive strain were applied to the chondrocytes encapsulated in alginate and gelatin gels after applying high HP at 25 MPa, which induced damage to the chondrocytes. It was found that compressive stimulation increased the expression of genes related to osteoarthritis. Furthermore, the simultaneous application of compressive strain and HP, which is similar to the physiological environment in cartilage, had an inhibitory effect on the expression of genes related to osteoarthritis. HP alone also suppressed the expression of osteoarthritis-related genes. Therefore, the simultaneous hydrostatic and compressive stress-loading device developed to simulate the mechanical environment in vivo may be an important tool for elucidating the mechanisms of disease onset and homeostasis in cartilage.
Keywords: articular cartilage; bioreactor; mechanical stimulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

