Tris(pentafluorophenyl)borane-catalyzed Hydride Transfer Reactions in Polysiloxane Chemistry-Piers-Rubinsztajn Reaction and Related Processes
- PMID: 37630197
- PMCID: PMC10459531
- DOI: 10.3390/molecules28165941
Tris(pentafluorophenyl)borane-catalyzed Hydride Transfer Reactions in Polysiloxane Chemistry-Piers-Rubinsztajn Reaction and Related Processes
Abstract
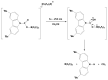
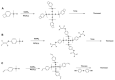
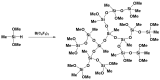
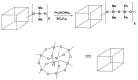
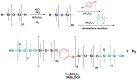
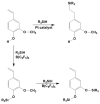
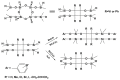
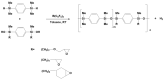
Tris(pentafluorophenyl)borane (TPFPB) is a unique Lewis acid that catalyzes the condensation between hydrosilanes (Si-H) and alkoxysilanes (Si-OR), leading to the formation of siloxane bonds (Si-OSi) with the release of hydrocarbon (R-H) as a byproduct-the so-called Piers-Rubinsztajn reaction. The analogous reactions of hydrosilanes with silanols (Si-OH), alcohols (R-OH), ethers (R-OR') or water in the presence of TPFPB leads to the formation of a siloxane bond, alkoxysilane (Si-OR or Si-OR') or silanol (Si-OH), respectively. The above processes, often referred to as Piers-Rubinsztajn reactions, provide new synthetic tools for the controlled synthesis of siloxane materials under mild conditions with high yields. The common feature of these reactions is the TPFPB-mediated hydride transfer from silicon to carbon or hydrogen. This review presents a summary of 20 years of research efforts related to this field, with a focus on new synthetic methodologies leading to numerous previously difficult to synthesize well-defined siloxane oligomers, polymers and copolymers of a complex structure and potential applications of these new materials. In addition, the mechanistic aspects of the recently discovered reactions involving hydride transfer from silicon to silicon are discussed in more detail.
Keywords: Piers–Rubinsztajn reaction; dehydrocarbonative condensation; dehydrogenative condensation; hydride transfer polymerization; mechanism; tris(pentafluorophenyl)borane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









































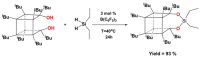












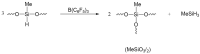
References
-
- Butts M., Cella J., Wood C.D., Gillette G., Kerboua R., Leman J., Lewis L., Rubinsztajn S., Schattenmann F., Stein J. Encyclopedia of Polymer Science and Technology. 4th ed. Volume 12. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2014. Silicones; pp. 464–541.
-
- Mordor Intelligence Research & Advisory. Silicone Market Size & Share Analysis—Growth Trends & Forecasts (2023–2028). Mordor Intelligence, 2023. [(accessed on 22 July 2023)]. Available online: https://www.mordorintelligence.com/industry-reports/silicone-market.
-
- Kipping F. Organic derivative of silicon. Preparation of alkyl silicon chlorides. Proc. Chem. Soc. 1904;20:15.
-
- Liebhafsky H.A., Liebhafsky S.S., Wise G. Silicones under the Monogram: A Story of Industrial Research. John Wiley & Sons; Hoboken, NJ, USA: 1978.
-
- Noll W. Chemistry and Technology of Silicones. Elsevier; Amsterdam, The Netherlands: 2012.
Publication types
LinkOut - more resources
Full Text Sources

