Anion Complexation by an Azocalix[4]arene Derivative and the Scope of Its Fluoride Complex Salt to Capture CO2 from the Air
- PMID: 37630281
- PMCID: PMC10458297
- DOI: 10.3390/molecules28166029
Anion Complexation by an Azocalix[4]arene Derivative and the Scope of Its Fluoride Complex Salt to Capture CO2 from the Air
Abstract
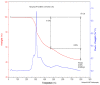
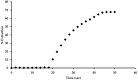
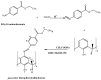
A newly synthesized upper rim azocalix[4]arene, namely 5,11,17,23-tetra[(4-ethylacetoxyphenyl) (azo)]calix[4]arene, CA-AZ has been fully characterized, and its chromogenic and selective properties for anions are reported. Among univalent anions, the receptor is selective for the fluoride anion, and its mode of interaction in solution is discussed. The kinetics of the complexation process were found to be very fast as reflected in the immediate colour change observed with a naked eye resulting from the receptor-anion interaction. An emphasis is made about the relevance in selecting a solvent in which the formulation of the process is representative of the events taking place in the solution. The composition of the fluoride complex investigated using UV/VIS spectrophotometry, conductance measurements and titration calorimetry was 1:1, and the thermodynamics of complexation of anions and CA-AZ in DMSO were determined. The fluoride complex salt was isolated, and a detailed investigation was carried out to assess its ability to remove CO2 from the air. The recycling of the complex was easily achieved. Final conclusions are given.
Keywords: azocalix[4]arene; carbon dioxide capture; fluoride complex salt.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Schrag P.D. Preparing to capture Carbon. Science. 2007;315:812–813. - PubMed
-
- Li L., Zhao N., Wei W., Sun Y. A Review of research progress on CO2 capture, storage and utilization I Chinese Academy of Sciences. Fuel. 2013;108:112–130.
-
- Dawson R., Cooper A.I., Adams D.J. Chemistry functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 2013;62:335–522.
-
- Perry S.F., Abdallah S. Mechanisms and consequences of carbon dioxide sensing in fish. Respir. Phys. Neurobiol. 2012;184:309–315. - PubMed
-
- Neethirajan S., Jayas D.S., Sadistap S. Carbon dioxide (CO2) sensors for the Agri-Food Industry—A Review. Food Bioprocess Technol. 2009;2:115–121.
Grants and funding
LinkOut - more resources
Full Text Sources

