Bismacrocycle: Structures and Applications
- PMID: 37630294
- PMCID: PMC10458016
- DOI: 10.3390/molecules28166043
Bismacrocycle: Structures and Applications
Abstract
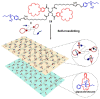
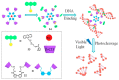
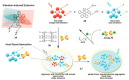
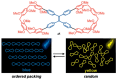
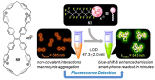
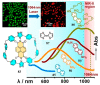
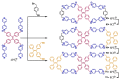
In the past half-century, macrocycles with different structures and functions, have played a critical role in supramolecular chemistry. Two macrocyclic moieties can be linked to form bismacrocycle molecules. Compared with monomacrocycle, the unique structures of bismacrocycles led to their specific recognition and assembly properties, also a wide range of applications, including molecular recognition, supramolecular self-assembly, advanced optical material construction, etc. In this review, we focus on the structure of bismacrocycle and their applications. Our goal is to summarize and outline the possible future development directions of bismacrocycle research.
Keywords: advanced optical materials; applications; bismacrocycle; self-assembly; supramolecular chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















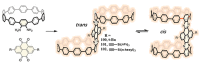




References
-
- Gokel M.R., McKeever M., Meisel J.W., Negin S., Patel M.B., Yin S., Gokel G.W. Crown ethers having side arms: A diverse and versatile supramolecular chemistry. J. Coord. Chem. 2021;74:14–39. doi: 10.1080/00958972.2021.1878352. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

