Binding Specificity of a Novel Cyclo/Maltodextrin-Binding Protein and Its Role in the Cyclodextrin ABC Importer System from Thermoanaerobacterales
- PMID: 37630332
- PMCID: PMC10458862
- DOI: 10.3390/molecules28166080
Binding Specificity of a Novel Cyclo/Maltodextrin-Binding Protein and Its Role in the Cyclodextrin ABC Importer System from Thermoanaerobacterales
Abstract
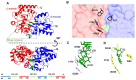
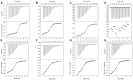
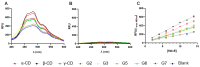
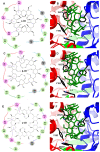
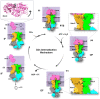
Extracellular synthesis of functional cyclodextrins (CDs) as intermediates of starch assimilation is a convenient microbial adaptation to sequester substrates, increase the half-life of the carbon source, carry bioactive compounds, and alleviate chemical toxicity through the formation of CD-guest complexes. Bacteria encoding the four steps of the carbohydrate metabolism pathway via cyclodextrins (CM-CD) actively internalize CDs across the microbial membrane via a putative type I ATP-dependent ABC sugar importer system, MdxEFG-(X/MsmX). While the first step of the CM-CD pathway encompasses extracellular starch-active cyclomaltodextrin glucanotransferases (CGTases) to synthesize linear dextrins and CDs, it is the ABC importer system in the second step that is the critical factor in determining which molecules from the CGTase activity will be internalized by the cell. Here, structure-function relationship studies of the cyclo⁄maltodextrin-binding protein MdxE of the MdxEFG-MsmX importer system from Thermoanaerobacter mathranii subsp. mathranii A3 are presented. Calorimetric and fluorescence studies of recombinant MdxE using linear dextrins and CDs showed that although MdxE binds linear dextrins and CDs with high affinity, the open-to-closed conformational change is solely observed after α- and β-CD binding, suggesting that the CM-CD pathway from Thermoanaerobacterales is exclusive for cellular internalization of these molecules. Structural analysis of MdxE coupled with docking simulations showed an overall architecture typically found in sugar-binding proteins (SBPs) that comprised two N- and C-domains linked by three small hinge regions, including the conserved aromatic triad Tyr193/Trp269/Trp378 in the C-domain and Phe87 in the N-domain involved in CD recognition and stabilization. Structural bioinformatic analysis of the entire MdxFG-MsmX importer system provided further insights into the binding, internalization, and delivery mechanisms of CDs. Hence, while the MdxE-CD complex couples to the permease subunits MdxFG to deliver the CD into the transmembrane channel, the dimerization of the cytoplasmatic promiscuous ATPase MsmX triggers active transport into the cytoplasm. This research provides the first results on a novel thermofunctional SBP and its role in the internalization of CDs in extremely thermophilic bacteria.
Keywords: CM-CD; MdxE; SBP; carbohydrate metabolism; starch-converting pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Shim J.-H., Park J.-T., Hong J.-S., Kim K.W., Kim M.-J., Auh J.-H., Kim Y.-W., Park C.-S., Boos W., Kim J.-W., et al. Role of Maltogenic Amylase and Pullulanase in Maltodextrin and Glycogen Metabolism of Bacillus subtilis 168. J. Bacteriol. 2009;191:4835–4844. doi: 10.1128/JB.00176-09. - DOI - PMC - PubMed
-
- Grégorio C. A Review: A History of Cyclodextrins. Chem. Rev. 2014;114:10940–10975. - PubMed
-
- Uitdehaag J.C., van der Veen B.A., Dijkhuizen L., Dijkstra B.W. Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-amylase family. Enzym. Microb. Technol. 2002;30:295–304. doi: 10.1016/S0141-0229(01)00498-7. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

