Dabcyl as a Naked Eye Colorimetric Chemosensor for Palladium Detection in Aqueous Medium
- PMID: 37630363
- PMCID: PMC10459738
- DOI: 10.3390/molecules28166111
Dabcyl as a Naked Eye Colorimetric Chemosensor for Palladium Detection in Aqueous Medium
Abstract
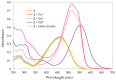
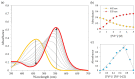
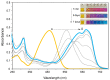
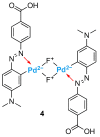
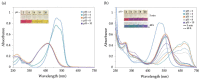
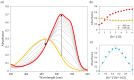
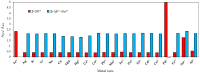
Industrial activity has raised significant concerns regarding the widespread pollution caused by metal ions, contaminating ecosystems and causing adverse effects on human health. Therefore, the development of sensors for selective and sensitive detection of these analytes is extremely important. In this regard, an azo dye, Dabcyl 2, was synthesised and investigated for sensing metal ions with environmental and industrial relevance. The cation binding character of 2 was evaluated by colour changes as seen by the naked eye, UV-Vis and 1H NMR titrations in aqueous mixtures of SDS (0.02 M, pH 6) solution with acetonitrile (99:1, v/v). Out of the several cations tested, chemosensor 2 had a selective response for Pd2+, Sn2+ and Fe3+, showing a remarkable colour change visible to the naked eye and large bathochromic shifts in the UV-Vis spectrum of 2. This compound was very sensitive for Pd2+, Sn2+ and Fe3+, with a detection limit as low as 5.4 × 10-8 M, 1.3 × 10-7 M and 5.2 × 10-8 M, respectively. Moreover, comparative studies revealed that chemosensor 2 had high selectivity towards Pd2+ even in the presence of other metal ions in SDS aqueous mixtures.
Keywords: Dabcyl; azo dye; colorimetric chemosensor; iron; naked eye; palladium; tin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Balamurugan R., Liu J.-H., Liu B.-T. A review of recent developments in fluorescent sensors for the selective detection of palladium ions. Coord. Chem. Rev. 2018;376:196–224. doi: 10.1016/j.ccr.2018.07.017. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

