Structural Studies of Monounsaturated and ω-3 Polyunsaturated Free Fatty Acids in Solution with the Combined Use οf NMR and DFT Calculations-Comparison with the Liquid State
- PMID: 37630396
- PMCID: PMC10459368
- DOI: 10.3390/molecules28166144
Structural Studies of Monounsaturated and ω-3 Polyunsaturated Free Fatty Acids in Solution with the Combined Use οf NMR and DFT Calculations-Comparison with the Liquid State
Abstract
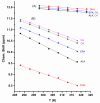
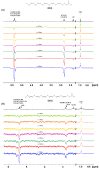
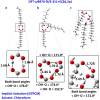
Molecular structures, in chloroform and DMSO solution, of the free fatty acids (FFAs) caproleic acid, oleic acid, α-linolenic acid, eicosapentanoic acid (EPA) and docosahexaenoic acid (DHA) are reported with the combined use of NMR and DFT calculations. Variable temperature and concentration chemical shifts of the COOH protons, transient 1D NOE experiments and DFT calculations demonstrate the major contribution of low molecular weight aggregates of dimerized fatty acids through intermolecular hydrogen bond interactions of the carboxylic groups, with parallel and antiparallel interdigitated structures even at the low concentration of 20 mM in CDCl3. For the dimeric DHA, a structural model of an intermolecular hydrogen bond through carboxylic groups and an intermolecular hydrogen bond between the carboxylic group of one molecule and the ω-3 double bond of a second molecule is shown to play a role. In DMSO-d6 solution, NMR and DFT studies show that the carboxylic groups form strong intermolecular hydrogen bond interactions with a single discrete solvation molecule of DMSO. These solvation species form parallel and antiparallel interdigitated structures of low molecular weight, as in chloroform solution. This structural motif, therefore, is an intrinsic property of the FFAs, which is not strongly affected by the length and degree of unsaturation of the chain and the hydrogen bond ability of the solvent.
Keywords: 1D 1H NOE; 1H NMR chemical shift; ALA; DFT; DHA; EPA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gunstone F.D. Fatty Acid and Lipid Chemistry. 1st ed. Springer; New York, NY, USA: 1996.
-
- Vance D.E., Vance J.E. Biochemistry of Lipids, Lipoproteins and Membranes (New Comprehensive Biochemistry) 5th ed. Elsevier; Amsterdam, The Netherlands: 2008.
-
- Akoh C.C. Min. D.B. Food Lipids, Chemistry, Nutrition and Biochemistry. 2nd ed. Marcel Dekker Inc.; New York, NY, USA: 2002.
-
- Leray C. Dietary Lipids for Healthy Brain Function. CRC Press; Boca Raton, FL, USA: 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

