Preclinical Evaluation of a New Series of Albumin-Binding 177Lu-Labeled PSMA-Based Low-Molecular-Weight Radiotherapeutics
- PMID: 37630410
- PMCID: PMC10459686
- DOI: 10.3390/molecules28166158
Preclinical Evaluation of a New Series of Albumin-Binding 177Lu-Labeled PSMA-Based Low-Molecular-Weight Radiotherapeutics
Abstract
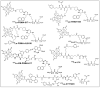
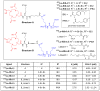
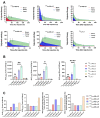
Prostate-specific membrane antigen (PSMA)-based low-molecular-weight agents using beta(β)-particle-emitting radiopharmaceuticals is a new treatment paradigm for patients with metastatic castration-resistant prostate cancer. Although results have been encouraging, there is a need to improve the tumor residence time of current PSMA-based radiotherapeutics. Albumin-binding moieties have been used strategically to enhance the tumor uptake and retention of existing PSMA-based investigational agents. Previously, we developed a series of PSMA-based, β-particle-emitting, low-molecular-weight compounds. From this series, 177Lu-L1 was selected as the lead agent because of its reduced off-target radiotoxicity in preclinical studies. The ligand L1 contains a PSMA-targeting Lys-Glu urea moiety with an N-bromobenzyl substituent in the ε-amino group of Lys. Here, we structurally modified 177Lu-L1 to improve tumor targeting using two known albumin-binding moieties, 4-(p-iodophenyl) butyric acid moiety (IPBA) and ibuprofen (IBU), and evaluated the effects of linker length and composition. Six structurally related PSMA-targeting ligands (Alb-L1-Alb-L6) were synthesized based on the structure of 177Lu-L1. The ligands were assessed for in vitro binding affinity and were radiolabeled with 177Lu following standard protocols. All 177Lu-labeled analogs were studied in cell uptake and selected cell efficacy studies. In vivo pharmacokinetics were investigated by conducting tissue biodistribution studies for 177Lu-Alb-L2-177Lu-Alb-L6 (2 h, 24 h, 72 h, and 192 h) in male NSG mice bearing human PSMA+ PC3 PIP and PSMA- PC3 flu xenografts. Preliminary therapeutic ratios of the agents were estimated from the area under the curve (AUC0-192h) of the tumors, blood, and kidney uptake values. Compounds were obtained in >98% radiochemical yields and >99% purity. PSMA inhibition constants (Kis) of the ligands were in the ≤10 nM range. The long-linker-based agents, 177Lu-Alb-L4 and 177Lu-Alb-L5, displayed significantly higher tumor uptake and retention (p < 0.001) than the short-linker-bearing 177Lu-Alb-L2 and 177Lu-Alb-L3 and a long polyethylene glycol (PEG) linker-bearing agent, 177Lu-Alb-L6. The area under the curve (AUC0-192h) of the PSMA+ PC3 PIP tumor uptake of 177Lu-Alb-L4 and 177Lu-Alb-L5 were >4-fold higher than 177Lu-Alb-L2, 177Lu-Alb-L3, and 177Lu-Alb-L6, respectively. Also, the PSMA+ PIP tumor uptake (AUC0-192h) of 177Lu-Alb-L2 and 177Lu-Alb-L3 was ~1.5-fold higher than 177Lu-Alb-L6. However, the lowest blood AUC0-192h and kidney AUC0-192h were associated with 177Lu-Alb-L6 from the series. Consequently, 177Lu-Alb-L6 displayed the highest ratios of AUC(tumor)-to-AUC(blood) and AUC(tumor)-to-AUC(kidney) values from the series. Among the other agents, 177Lu-Alb-L4 demonstrated a nearly similar ratio of AUC(tumor)-to-AUC(blood) as 177Lu-Alb-L6. The tumor-to-blood ratio was the dose-limiting therapeutic ratio for all of the compounds. Conclusions: 177Lu-Alb-L4 and 177Lu-Alb-L6 showed high tumor uptake in PSMA+ tumors and tumor-to-blood ratios. The data suggest that linker length and composition can be modulated to generate an optimized therapeutic agent.
Keywords: albumin; linker; prostate cancer; prostate-specific membrane antigen; radiopharmaceutical therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile.Mol Pharm. 2018 Mar 5;15(3):934-946. doi: 10.1021/acs.molpharmaceut.7b00877. Epub 2018 Feb 5. Mol Pharm. 2018. PMID: 29400475
-
Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties To Improve Prostate Cancer Therapy.Mol Pharm. 2018 Jun 4;15(6):2297-2306. doi: 10.1021/acs.molpharmaceut.8b00152. Epub 2018 May 2. Mol Pharm. 2018. PMID: 29684274
-
Preclinical Evaluation of 203/212Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer.J Nucl Med. 2020 Jan;61(1):80-88. doi: 10.2967/jnumed.119.229393. Epub 2019 Jun 28. J Nucl Med. 2020. PMID: 31253744 Free PMC article.
-
Preclinical Development in Radiopharmaceutical Therapy for Prostate Cancer.Semin Nucl Med. 2023 Sep;53(5):663-686. doi: 10.1053/j.semnuclmed.2023.06.007. Epub 2023 Jul 18. Semin Nucl Med. 2023. PMID: 37468417 Review.
-
Development of radioligands with an albumin-binding moiety of 4-(P-Iodophenyl) butyric acid for theranostic applications.J Control Release. 2025 Jun 10;382:113757. doi: 10.1016/j.jconrel.2025.113757. Epub 2025 Apr 20. J Control Release. 2025. PMID: 40262707 Review.
Cited by
-
Physiologically based radiopharmacokinetic (PBRPK) modeling to simulate and analyze radiopharmaceutical therapies: studies of non-linearities, multi-bolus injections, and albumin binding.EJNMMI Radiopharm Chem. 2024 Jan 22;9(1):6. doi: 10.1186/s41181-023-00236-w. EJNMMI Radiopharm Chem. 2024. PMID: 38252191 Free PMC article.
-
Preclinical studies of a PARP-targeted theranostic radiopharmaceutical for pancreatic cancer.Front Pharmacol. 2025 May 15;16:1576587. doi: 10.3389/fphar.2025.1576587. eCollection 2025. Front Pharmacol. 2025. PMID: 40444043 Free PMC article.
-
Radionuclide-Labeled Biomaterials: A Novel Strategy for Tumor-Targeted Therapy.Biomimetics (Basel). 2025 Jun 11;10(6):394. doi: 10.3390/biomimetics10060394. Biomimetics (Basel). 2025. PMID: 40558363 Free PMC article. Review.
-
Fluorescent PSMA-Targeted Radiotheranostic Compounds for Multiscale Imaging.Bioconjug Chem. 2025 Jul 16;36(7):1448-1460. doi: 10.1021/acs.bioconjchem.5c00139. Epub 2025 Jun 27. Bioconjug Chem. 2025. PMID: 40574660
-
Design and Preclinical Evaluation of a Novel Prostate-Specific Membrane Antigen Radioligand Modified with a Transthyretin Binder.Cancers (Basel). 2024 Mar 23;16(7):1262. doi: 10.3390/cancers16071262. Cancers (Basel). 2024. PMID: 38610940 Free PMC article.
References
-
- Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

