Comparative Longitudinal Serological Study of Anti-SARS-CoV-2 Antibody Profiles in People with COVID-19
- PMID: 37630545
- PMCID: PMC10458948
- DOI: 10.3390/microorganisms11081985
Comparative Longitudinal Serological Study of Anti-SARS-CoV-2 Antibody Profiles in People with COVID-19
Abstract
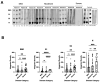
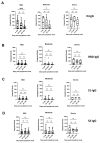
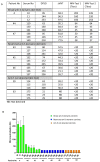
Serological diagnostic assays are essential tools for determining an individual's protection against viruses like SARS-CoV-2, tracking the spread of the virus in the community, and evaluating population immunity. To assess the diversity and quality of the anti-SARS-CoV-2 antibody response, we have compared the antibody profiles of people with mild, moderate, and severe COVID-19 using a dot blot assay. The test targeted the four major structural proteins of SARS-CoV-2, namely the nucleocapsid (N), spike (S) protein domains S1 and S2, and receptor-binding domain (RBD). Serum samples were collected from 63 participants at various time points for up to 300 days after disease onset. The dot blot assay revealed patient-specific differences in the anti-SARS-CoV-2 antibody profiles. Out of the 63 participants with confirmed SARS-CoV-2 infections and clinical COVID-19, 35/63 participants exhibited diverse and robust responses against the tested antigens, while 14/63 participants displayed either limited responses to a subset of antigens or no detectable antibody response to any of the antigens. Anti-N-specific antibody levels decreased within 300 days after disease onset, whereas anti-S-specific antibodies persisted. The dynamics of the antibody response did not change during the test period, indicating stable antibody profiles. Among the participants, 28/63 patients with restricted anti-S antibody profiles or undetectable anti-S antibody levels in the dot blot assay also exhibited weak neutralization activity, as measured by a surrogate virus neutralization test (sVNT) and a microneutralization test. These results indicate that in some cases, natural infections do not lead to the production of neutralizing antibodies. Furthermore, the study revealed significant serological variability among patients, regardless of the severity of their COVID-19 illness. These differences need to be carefully considered when evaluating the protective antibody status of individuals who have experienced primary SARS-CoV-2 infections.
Keywords: SARS-CoV-2; antibody profile; polyclonal durability; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Grant M.C., Geoghegan L., Arbyn M., Mohammed Z., McGuinness L., Clarke E.L., Wade R.G. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS ONE. 2020;15:e0234765. doi: 10.1371/journal.pone.0234765. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention (CDCP) Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases; Atlanta, GA, USA: 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

