Native Pig Neutrophil Products: Insights into Their Antimicrobial Activity
- PMID: 37630679
- PMCID: PMC10459379
- DOI: 10.3390/microorganisms11082119
Native Pig Neutrophil Products: Insights into Their Antimicrobial Activity
Abstract
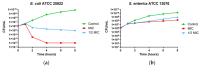
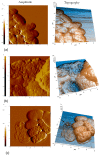
Cationic antimicrobial peptides are molecules with potential applications for treating infections due to their antimicrobial and immunomodulatory properties. The aim of this work was to explore the antimicrobial activity and mechanisms of action of a porcine neutrophil cathelicidin mixture (MPPN). Gram-positive and Gram-negative bacteria were used to determine the minimum inhibitory concentration (MIC) and experiments of both time-kill kinetics and effects on growth curves were performed. Planar black lipid bilayer conductance was measured to analyze the interaction of MPPN with lipid bilayers. Visualization of bacterial surfaces and membrane alterations was achieved using atomic force microscopy and transmission electron microscopy. The effects on the activity of efflux pumps (EPs) were studied with an intracellular accumulation of acridine orange (AO) assay. In E. coli, MPPN behaves as a bactericide at high concentrations and as a bacteriostatic at lower concentrations. The bacteriostatic effect was also observed for slightly shorter periods in S. enterica. The mixture was not active on S. aureus. The increase in AO accumulation in the presence of MPPN indicates that, at least in E. coli, the mixture causes inhibition of the EP function. Observed and detected variable conductance events demonstrate a strong MPPN effect on lipid bilayers. Damage to the structure of treated E. coli indicates that MPPN induces alterations in the bacterial surface. The use of AMPs capable of inhibiting EP can be seen as a good tool to combat antimicrobial resistance since they could be used alone or in combination with other conventional antibiotics to which bacteria have become resistant.
Keywords: antimicrobial peptides; atomic force microscopy; black lipid bilayer; cathelicidins; efflux pumps; membrane disruption; transmission electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

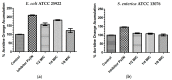
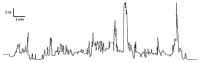

References
-
- Haney E.F., Mansour S.C., Hancock R.E.W. Methods in Molecular Biology. Volume 1548. Humana Press Inc.; Totowa, NJ, USA: 2017. Antimicrobial Peptides: An Introduction; pp. 3–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

