Monocarboxylate Transporter 13 (MCT13/SLC16A13) Functions as a Novel Plasma Membrane Oligopeptide Transporter
- PMID: 37630718
- PMCID: PMC10458055
- DOI: 10.3390/nu15163527
Monocarboxylate Transporter 13 (MCT13/SLC16A13) Functions as a Novel Plasma Membrane Oligopeptide Transporter
Abstract
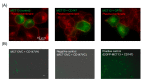
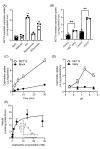
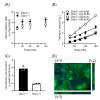
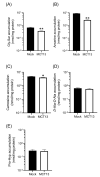
SLC16A13, which encodes the monocarboxylate transporter 13 (MCT13), is a susceptibility gene for type 2 diabetes and is expressed in the liver and duodenum. Some peptidase-resistant oligopeptides are absorbed in the gastrointestinal tract and affect glycemic control in the body. Their efficient absorption is mediated by oligopeptide transporter(s) at the apical and basolateral membranes of the intestinal epithelia; however, the molecules responsible for basolateral oligopeptide transport have not been identified. In this study, we examined whether MCT13 functions as a novel basolateral oligopeptide transporter. We evaluated the uptake of oligopeptides and peptidomimetics in MCT13-transfected cells. The uptake of cephradine, a probe for peptide transport system(s), significantly increased in MCT13-transfected cells, and this increase was sensitive to membrane potential. The cellular accumulation of bioactive peptides, such as anserine and carnosine, was decreased by MCT13, indicating MCT13-mediated efflux transport activity. In polarized Caco-2 cells, MCT13 was localized at the basolateral membrane. MCT13 induction enhanced cephradine transport in an apical-to-basal direction across Caco-2 cells. These results indicate that MCT13 functions as a novel efflux transporter of oligopeptides and peptidomimetics, driven by electrochemical gradients across the plasma membrane, and it may be involved in the transport of these compounds across the intestinal epithelia.
Keywords: CD147; cephradine; intestinal epithelium; membrane potential; membrane transport; monocarboxylate transporter 13; oligopeptide; protein–protein interaction; transporter.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures


Similar articles
-
Metabolism, uptake, and transepithelial transport of the stereoisomers of Val-Val-Val in the human intestinal cell line, Caco-2.Pharm Res. 1996 Nov;13(11):1663-7. doi: 10.1023/a:1016436606183. Pharm Res. 1996. PMID: 8956331
-
Transepithelial transport of oral cephalosporins by monolayers of intestinal epithelial cell line Caco-2: specific transport systems in apical and basolateral membranes.J Pharmacol Exp Ther. 1992 Apr;261(1):195-201. J Pharmacol Exp Ther. 1992. PMID: 1560365
-
Transcellular transport of oral cephalosporins in human intestinal epithelial cells, Caco-2: interaction with dipeptide transport systems in apical and basolateral membranes.J Pharmacol Exp Ther. 1994 Aug;270(2):498-504. J Pharmacol Exp Ther. 1994. PMID: 8071843
-
[Analysis of Endogenous Metabolite Transporters for the Development of Novel Pharmaceutical Therapies and Drug Targets].Yakugaku Zasshi. 2024;144(12):1051-1054. doi: 10.1248/yakushi.24-00130. Yakugaku Zasshi. 2024. PMID: 39617467 Review. Japanese.
-
The oligopeptide transporter (Pept-1) in human intestine: biology and function.Gastroenterology. 1997 Jul;113(1):332-40. doi: 10.1016/s0016-5085(97)70112-4. Gastroenterology. 1997. PMID: 9207295 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

