Anticancer Effect of Gallic Acid on Acidity-Induced Invasion of MCF7 Breast Cancer Cells
- PMID: 37630786
- PMCID: PMC10458441
- DOI: 10.3390/nu15163596
Anticancer Effect of Gallic Acid on Acidity-Induced Invasion of MCF7 Breast Cancer Cells
Abstract
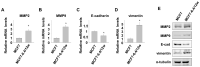
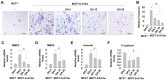
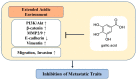
The acidic tumor environment has emerged as a crucial factor influencing the metastatic potential of cancer. We investigated the effect of an acidic environment on the acquisition of metastatic properties in MCF7 breast cancer cells and explored the inhibitory effects of gallic acid. Prolonged exposure to acidic culture conditions (over 12 weeks at pH 6.4) induced the acquisition of migratory and invasive properties in MCF7 cells, accompanied by increased expression of Matrix Metalloproteinase 2 and 9 (MMP2 and MMP9, respectively), together with alterations in E-cadherin, vimentin, and epithelial-to-mesenchymal transition markers. Gallic acid effectively inhibited the survival of acidity-adapted MCF7 (MCF7-6.4/12w) cells at high concentrations (>30 μM) and reduced metastatic characteristics induced by acidic conditions at low concentration ranges (5-20 μM). Moreover, gallic acid suppressed the PI3K/Akt pathway and the nuclear accumulation of β-catenin, which were elevated in MCF7-6.4/12w cells. These findings highlight the potential of gallic acid as a promising therapeutic agent for metastatic traits in breast cancer cells under acidic conditions.
Keywords: MCF7 cells; acidic tumor environment; gallic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

