Aspartame Safety as a Food Sweetener and Related Health Hazards
- PMID: 37630817
- PMCID: PMC10459792
- DOI: 10.3390/nu15163627
Aspartame Safety as a Food Sweetener and Related Health Hazards
Abstract
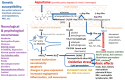
Aspartame is the methyl-ester of the aspartate-phenylalanine dipeptide. Over time, it has become a very popular artificial sweetener. However, since its approval by the main food safety agencies, several concerns have been raised related to neuropsychiatric effects and neurotoxicity due to its ability to activate glutamate receptors, as well as carcinogenic risks due to the increased production of reactive oxygen species. Within this review, we critically evaluate reports concerning the safety of aspartame. Some studies evidenced subtle mood and behavioral changes upon daily high-dose intake below the admitted limit. Epidemiology studies also evidenced associations between daily aspartame intake and a higher predisposition for malignant diseases, like non-Hodgkin lymphomas and multiple myelomas, particularly in males, but an association by chance still could not be excluded. While the debate over the carcinogenic risk of aspartame is ongoing, it is clear that its use may pose some dangers in peculiar cases, such as patients with seizures or other neurological diseases; it should be totally forbidden for patients with phenylketonuria, and reduced doses or complete avoidance are advisable during pregnancy. It would be also highly desirable for every product containing aspartame to clearly indicate on the label the exact amount of the substance and some risk warnings.
Keywords: artificial sweetener; aspartame; carcinogenic risk; excitotoxicity; neuropsychiatric symptoms; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Possible neurologic effects of aspartame, a widely used food additive.Environ Health Perspect. 1987 Nov;75:53-7. doi: 10.1289/ehp.877553. Environ Health Perspect. 1987. PMID: 3319565 Free PMC article. Review.
-
Aspartame: review of safety.Regul Toxicol Pharmacol. 2002 Apr;35(2 Pt 2):S1-93. doi: 10.1006/rtph.2002.1542. Regul Toxicol Pharmacol. 2002. PMID: 12180494 Review.
-
Can aspartame meet our expectations?J Am Diet Assoc. 1983 Aug;83(2):142-6. J Am Diet Assoc. 1983. PMID: 6348131 Review.
-
Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women.Am J Clin Nutr. 2012 Dec;96(6):1419-28. doi: 10.3945/ajcn.111.030833. Epub 2012 Oct 24. Am J Clin Nutr. 2012. PMID: 23097267 Free PMC article.
-
Neurobehavioral effects of aspartame consumption.Res Nurs Health. 2014 Jun;37(3):185-93. doi: 10.1002/nur.21595. Epub 2014 Apr 3. Res Nurs Health. 2014. PMID: 24700203 Free PMC article.
Cited by
-
Development of Thin Film Microextraction with Natural Deep Eutectic Solvents as 'Eutectosorbents' for Preconcentration of Popular Sweeteners and Preservatives from Functional Beverages and Flavoured Waters.Molecules. 2024 Sep 26;29(19):4573. doi: 10.3390/molecules29194573. Molecules. 2024. PMID: 39407502 Free PMC article.
-
Dismantling the myth of "all foods fit" in eating disorder treatment.J Eat Disord. 2024 May 17;12(1):60. doi: 10.1186/s40337-024-01017-9. J Eat Disord. 2024. PMID: 38760858 Free PMC article.
-
Sweet or sour? A review of the Aspartame market landscape, carcinogenicity, and its socioeconomic impact.J Food Sci Technol. 2025 Jan;62(1):24-37. doi: 10.1007/s13197-024-06077-y. Epub 2024 Sep 6. J Food Sci Technol. 2025. PMID: 39867619 Review.
-
Rediscovering Sweetness: The Evolution and Impact of Non-Nutritive and Natural Sweeteners.Curr Nutr Rep. 2025 Apr 2;14(1):54. doi: 10.1007/s13668-025-00646-z. Curr Nutr Rep. 2025. PMID: 40175668 Review.
-
Aspartame and Human Health: A Mini-Review of Carcinogenic and Systemic Effects.J Xenobiot. 2025 Jul 7;15(4):114. doi: 10.3390/jox15040114. J Xenobiot. 2025. PMID: 40700161 Free PMC article. Review.
References
-
- Mazur R.H. Discovery of aspartame. In: Stegink L.D., Filer L.J.J., editors. Aspartame: Physiology and Biochemistry. Marcel Dekker; New York, NY, USA: 1984. pp. 3–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

