Probiotic OMNi-BiOTiC® 10 AAD Reduces Cyclophosphamide-Induced Inflammation and Adipose Tissue Wasting in Mice
- PMID: 37630845
- PMCID: PMC10458463
- DOI: 10.3390/nu15163655
Probiotic OMNi-BiOTiC® 10 AAD Reduces Cyclophosphamide-Induced Inflammation and Adipose Tissue Wasting in Mice
Abstract
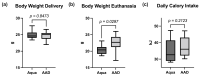
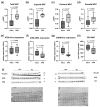
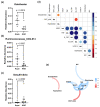
Cancer therapy is often associated with severe side effects such as drug induced weight loss, also known as chemotherapy-induced cachexia. The aim of this study was to investigate the effects of a multispecies probiotic (OMNi-BiOTiC® 10 AAD) in a chemotherapy mouse model. A total of 24 male BALB/c mice were gavage-fed with the probiotic formulation or water, once a day for 3 weeks. In the third week, the mice received intraperitoneal cyclophosphamide. At euthanasia, the organs were dissected, and serum was sampled for cytokine analysis. Tight junction components, myosin light chain kinase, mucins, and apoptosis markers were detected in the ileum and colon using histological analyses and qRT-PCR. Lipolysis was analyzed by enzymatic activity assay, Western blotting analyses, and qRT-PCR in WAT. The fecal microbiome was measured with 16S-rRNA gene sequencing from stool samples, and fecal volatile organic compounds analysis was performed using gas chromatography/mass spectrometry. The probiotic-fed mice exhibited significantly less body weight loss and adipose tissue wasting associated with a reduced CGI58 mediated lipolysis. They showed significantly fewer pro-inflammatory cytokines and lower gut permeability compared to animals fed without the probiotic. The colons of the probiotic-fed animals showed lower inflammation scores and less goblet cell loss. qRT-PCR revealed no differences in regards to tight junction components, mucins, or apoptosis markers. No differences in microbiome alpha diversity, but differences in beta diversity, were observed between the treatment groups. Taxonomic analysis showed that the probiotic group had a lower relative abundance of Odoribacter and Ruminococcus-UCG014 and a higher abundance of Desulfovibrio. VOC analysis yielded no significant differences. The results of this study indicate that oral administration of the multispecies probiotic OMNi-BiOTiC® 10 AAD could mitigate cyclophosphamide-induced chemotherapy side effects.
Keywords: chemotherapy; gut permeability; histomorphometry; inflammation; microbiome; volatile organic compound; weight loss.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures