Analysis of the Effect of the TRPC4/TRPC5 Blocker, ML204, in Sucrose-Induced Metabolic Imbalance
- PMID: 37631015
- PMCID: PMC10459798
- DOI: 10.3390/ph16081100
Analysis of the Effect of the TRPC4/TRPC5 Blocker, ML204, in Sucrose-Induced Metabolic Imbalance
Abstract
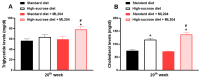
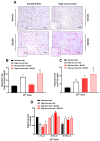
Sugar-induced metabolic imbalances are a major health problem since an excessive consumption of saccharides has been linked to greater obesity rates at a global level. Sucrose, a disaccharide composed of 50% glucose and 50% fructose, is commonly used in the food industry and found in a range of fast, restaurant, and processed foods. Herein, we investigated the effects of a TRPC4/TRPC5 blocker, ML204, in the metabolic imbalances triggered by early exposure to sucrose-enriched diet in mice. TRPC4 and TRPC5 belong to the family of non-selective Ca+2 channels known as transient receptor potential channels. High-sucrose (HS)-fed animals with hyperglycaemia and dyslipidaemia, were accompanied by increased body mass index. mesenteric adipose tissue accumulation with larger diameter cells and hepatic steatosis in comparison to those fed normal diet. HS mice also exhibited enhanced adipose, liver, and pancreas TNFα and VEGF levels. ML204 exacerbated hyperglycaemia, dyslipidaemia, fat tissue deposition, hepatic steatosis, and adipose tissue and liver TNFα in HS-fed mice. Normal mice treated with the blocker had greater hepatic steatosis and adipose tissue cell numbers/diameter than those receiving vehicle, but showed no significant changes in tissue inflammation, glucose, and lipid levels. The results indicate that TRPC4/TRPC5 protect against the metabolic imbalances caused by HS ingestion.
Keywords: TRPC4 and TRPC5 channels; fat deposition; hepatic steatosis; high sucrose intake; metabolic changes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Geidl-Flueck B., Hochuli M., Németh Á., Eberl A., Derron N., Köfeler H.C., Tappy L., Berneis K., Spinas G.A., Gerber P.A. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 2021;75:46–54. - PubMed
-
- Shetty A. Significance of sugar intake in young adults: A review. Int. J. Adolesc. Med. Health. 2021;33:375–378. - PubMed
Grants and funding
- grant numbers: 305676/2019-9 and 408053/2018-6 to E.S.F; 315072/2020-2 to V.M.-N.; and 302195/2022-0 to D.M-F/National Council for Scientific and Technological Development
- grant numbers: IECT-05738/18 to E.S.F.; and ACT-05691/21, and ACT 01784-21 to V.M.-N./Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão
- Programa Pró-Amazônia; grant number: 3325/2013; finance code 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- na/Instituto de Pesquisa Pelé Pequeno Príncipe
- na/INCT-INOVAMED
LinkOut - more resources
Full Text Sources

