Pregabalin-Tolperisone Combination to Treat Neuropathic Pain: Improved Analgesia and Reduced Side Effects in Rats
- PMID: 37631030
- PMCID: PMC10459435
- DOI: 10.3390/ph16081115
Pregabalin-Tolperisone Combination to Treat Neuropathic Pain: Improved Analgesia and Reduced Side Effects in Rats
Abstract
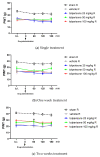
The current treatment of neuropathic pain (NP) is unsatisfactory; therefore, effective novel agents or combination-based analgesic therapies are needed. Herein, oral tolperisone, pregabalin, and duloxetine were tested for their antinociceptive effect against rat partial sciatic nerve ligation (pSNL)-induced tactile allodynia described by a decrease in the paw withdrawal threshold (PWT) measured by a dynamic plantar aesthesiometer. On day 7 after the operation, PWTs were assessed at 60, 120, and 180 min post-treatment. Chronic treatment was continued for 2 weeks, and again, PWTs were measured on day 14 and 21. None of the test compounds produced an acute antiallodynic effect. In contrast, after chronic treatment, tolperisone and pregabalin alleviated allodynia. In other experiments, on day 14, the acute antiallodynic effect of the tolperisone/pregabalin or duloxetine combination was measured. As a novel finding, a single dose of the tolperisone/pregabalin combination could remarkably alleviate allodynia acutely. It also restored the neuropathy-induced elevated CSF glutamate content. Furthermore, the combination is devoid of adverse effects related to motor and gastrointestinal transit functions. Tolperisone and pregabalin target voltage-gated sodium and calcium channels, respectively. The dual blockade effect of the combination might explain its advantageous acute analgesic effect in the present work.
Keywords: CSF glutamate content; allodynia; duloxetine; neuronal glutamate release; neuropathic pain; pregabalin; synaptosome; tolperisone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Kremer M., Yalcin I., Goumon Y., Wurtz X., Nexon L., Daniel D., Megat S., Ceredig R.A., Ernst C., Turecki G., et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J. Neurosci. 2018;38:9934–9954. doi: 10.1523/JNEUROSCI.1004-18.2018. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

