Cytokinin Promotes Jasmonic Acid Accumulation in the Control of Maize Leaf Growth
- PMID: 37631225
- PMCID: PMC10459232
- DOI: 10.3390/plants12163014
Cytokinin Promotes Jasmonic Acid Accumulation in the Control of Maize Leaf Growth
Abstract
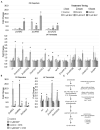
Plant organ growth results from the combined activity of cell division and cell expansion. The co-ordination of these two processes depends on the interplay between multiple hormones that determine the final organ size. Using the semidominant Hairy Sheath Frayed1 (Hsf1) maize mutant that hypersignals the perception of cytokinin (CK), we show that CK can reduce leaf size and growth rate by decreasing cell division. Linked to CK hypersignaling, the Hsf1 mutant has an increased jasmonic acid (JA) content, a hormone that can inhibit cell division. The treatment of wild-type seedlings with exogenous JA reduces maize leaf size and growth rate, while JA-deficient maize mutants have increased leaf size and growth rate. Expression analysis revealed the increased transcript accumulation of several JA pathway genes in the Hsf1 leaf growth zone. A transient treatment of growing wild-type maize shoots with exogenous CK also induced the expression of JA biosynthetic genes, although this effect was blocked by the co-treatment with cycloheximide. Together, our results suggest that CK can promote JA accumulation, possibly through the increased expression of specific JA pathway genes.
Keywords: cytokinin; jasmonic acid; maize.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

