Microneedles and Their Application in Transdermal Delivery of Antihypertensive Drugs-A Review
- PMID: 37631243
- PMCID: PMC10459756
- DOI: 10.3390/pharmaceutics15082029
Microneedles and Their Application in Transdermal Delivery of Antihypertensive Drugs-A Review
Abstract
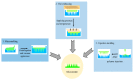
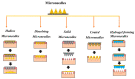
One of the most cutting-edge, effective, and least invasive pharmaceutical innovations is the utilization of microneedles (MNs) for drug delivery, patient monitoring, diagnostics, medicine or vaccine delivery, and other medical procedures (e.g., intradermal vaccination, allergy testing, dermatology, and blood sampling). The MN-based system offers many advantages, such as minimal cost, high medical effectiveness, comparatively good safety, and painless drug application. Drug delivery through MNs can possibly be viewed as a viable instrument for various macromolecules (e.g., proteins, peptides, and nucleic acids) that are not efficiently administered through traditional approaches. This review article provides an overview of MN-based research in the transdermal delivery of hypertensive drugs. The critical attributes of microneedles are discussed, including the mechanism of drug release, pharmacokinetics, fabrication techniques, therapeutic applications, and upcoming challenges. Furthermore, the therapeutic perspective and improved bioavailability of hypertensive drugs that are poorly aqueous-soluble are also discussed. This focused review provides an overview of reported studies and the recent progress of MN-based delivery of hypertensive drugs, paving the way for future pharmaceutical uses. As MN-based drug administration bypasses first-pass metabolism and the high variability in drug plasma levels, it has grown significantly more important for systemic therapy. In conclusion, MN-based drug delivery of hypertensive drugs for increasing bioavailability and patient compliance could support a new trend of hypertensive drug delivery and provide an alternative option, overcoming the restrictions of the current dosage forms.
Keywords: hypertension; lipid nanoparticles; microneedle; polymeric nanoparticles; transdermal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Erfanpoor S., Etemad K., Kazempour S., Hadaegh F., Hasani J., Azizi F., Parizadeh D., Khalili D. Diabetes, hypertension, and incidence of chronic kidney disease: Is there any multiplicative or additive interaction? Int. J. Endocrinol. Metab. 2021;19:e101061. doi: 10.5812/ijem.101061. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

