Evaluation of Strategies for Reducing Vancomycin-Piperacillin/Tazobactam Incompatibility
- PMID: 37631283
- PMCID: PMC10459903
- DOI: 10.3390/pharmaceutics15082069
Evaluation of Strategies for Reducing Vancomycin-Piperacillin/Tazobactam Incompatibility
Abstract
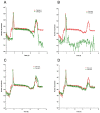
Background: Drug incompatibility is defined as a physical-chemical reaction between two or more injectable drugs and that results mainly in precipitation or insolubility. Several strategies for reducing incompatibilities have been implemented empirically in intensive care units. However, these strategies have never been compared directly (and particularly in terms of the particulate load and drug mass flow rate) under standardized conditions. The objective of the present in vitro study was to evaluate the impact of various strategies for preventing incompatibility between simultaneously infused vancomycin and piperacillin/tazobactam.
Methods: An in-line filter, a dilute vancomycin solution (5 mg/mL), and an alternative saline administration line were evaluated separately. The infusion line outlet was connected to a dynamic particle counter. The antibiotic concentration was measured in an HPLC-UV assay.
Result: The use of an in-line filter and an alternative saline administration route did not significantly reduce the particulate load caused by vancomycin-piperacillin/tazobactam incompatibility. Dilution of the vancomycin solution was associated with a significantly lower particulate load and maintenance of the vancomycin mass flow rate.
Discussion: It is important to systematically compare the efficacy of strategies for preventing drug incompatibility. The use of diluted vancomycin solution gave the best results in the case of vancomycin-piperacillin/tazobactam incompatibility.
Keywords: drug incompatibility; in-line filter; infusion; particulate load; piperacillin/tazobactam; vancomycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kanji S., Lam J., Johanson C., Singh A., Goddard R., Fairbairn J., Lloyd T., Monsour D., Kakal J. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit. Care Med. 2010;38:1890–1898. doi: 10.1097/CCM.0b013e3181e8adcc. - DOI - PubMed
LinkOut - more resources
Full Text Sources

