Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment
- PMID: 37631339
- PMCID: PMC10460031
- DOI: 10.3390/pharmaceutics15082124
Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment
Abstract
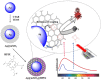
Malignant melanoma poses a significant global health burden. It is the most aggressive and lethal form of skin cancer, attributed to various risk factors such as UV radiation exposure, genetic modifications, chemical carcinogens, immunosuppression, and fair complexion. Photodynamic therapy is a promising minimally invasive treatment that uses light to activate a photosensitizer, resulting in the formation of reactive oxygen species, which ultimately promote cell death. When selecting photosensitizers for melanoma photodynamic therapy, the presence of melanin should be considered. Melanin absorbs visible radiation similar to most photosensitizers and has antioxidant properties, which undermines the reactive species generated in photodynamic therapy processes. These characteristics have led to further research for new photosensitizing platforms to ensure better treatment results. The development of photosensitizers has advanced with the use of nanotechnology, which plays a crucial role in enhancing solubility, optical absorption, and tumour targeting. This paper reviews the current approaches (that use the synergistic effect of different photosensitizers, nanocarriers, chemotherapeutic agents) in the photodynamic therapy of melanoma.
Keywords: melanoma; nanocarriers; non-porphyrin photosensitizers; photodynamic therapy; photosensitizer; porphyrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Radiation: Ultraviolet (UV) Radiation and Skin Cancer. [(accessed on 25 May 2023)]. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultra....
-
- Wagstaff W., Mwamba R.N., Grullon K., Armstrong M., Zhao P., Hendren-Santiago B., Qin K.H., Li A.J., Hu D.A., Youssef A., et al. Melanoma: Molecular Genetics, Metastasis, Targeted Therapies, Immunotherapies, and Therapeutic Resistance. Genes Dis. 2022;9:1608–1623. doi: 10.1016/j.gendis.2022.04.004. - DOI - PMC - PubMed
-
- Vicente A.L.S.A., Novoloaca A., Cahais V., Awada Z., Cuenin C., Spitz N., Carvalho A.L., Evangelista A.F., Crovador C.S., Reis R.M., et al. Cutaneous and Acral Melanoma Cross-OMICs Reveals Prognostic Cancer Drivers Associated with Pathobiology and Ultraviolet Exposure. Nat. Commun. 2022;13:4115. doi: 10.1038/s41467-022-31488-w. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

