Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol
- PMID: 37631342
- PMCID: PMC10459144
- DOI: 10.3390/pharmaceutics15082128
Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol
Abstract
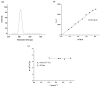
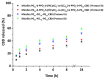
The present study describes the development of novel block copolymer nanocarriers of the phytocannabinoid cannabidiol (CBD), designed to enhance the solubility of the drug in water while achieving high encapsulation efficiency and prolonged drug release. Firstly, a well-defined amphiphilic block copolymer consisting of two outer hydrophilic polyglycidol (PG) blocks and a middle hydrophobic block of poly(ε-caprolactone) bearing pendant cinnamyl moieties (P(CyCL-co-CL)) were synthesized by the click coupling reaction of PG-monoalkyne and P(CyCL-co-CL)-diazide functional macroreagents. A non-modified polyglycidol/poly(ε-caprolactone) amphiphilic block copolymer was obtained as a referent system. Micellar carriers based on the two block copolymers were formed via the solvent evaporation method and loaded with CBD following two different protocols-loading during micelle formation and loading into preformed micelles. The key parameters/characteristics of blank and CBD-loaded micelles such as size, size distribution, zeta potential, molar mass, critical micelle concentration, morphology, and encapsulation efficiency were determined by using dynamic and static multiangle and electrophoretic light scattering, transmission electron microscopy, and atomic force microscopy. Embedding CBD into the micellar carriers affected their hydrodynamic radii to some extent, while the spherical morphology of particles was not changed. The nanoformulation based on the copolymer bearing cinnamyl moieties possessed significantly higher encapsulation efficiency and a slower rate of drug release than the non-modified copolymer. The comparative assessment of the antiproliferative effect of micellar CBD vs. the free drug against the acute myeloid leukemia-derived HL-60 cell line and Sezary Syndrome HUT-78 demonstrated that the newly developed systems have pronounced antitumor activity.
Keywords: cannabidiol; click reactions; functional nanomaterials; poly(ε-caprolactone); polyglycidol; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures









References
-
- Zhou W., Li C., Wang Z., Liu J. Factors affecting the stability of drug-loaded polymeric micelles and strategies for improvement. J. Nanoparticle Res. 2016;18:275. doi: 10.1007/s11051-016-3583-y. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

