Improving Riparin-A Dissolution through a Laponite Based Nanohybrid
- PMID: 37631350
- PMCID: PMC10458571
- DOI: 10.3390/pharmaceutics15082136
Improving Riparin-A Dissolution through a Laponite Based Nanohybrid
Abstract
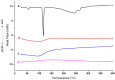
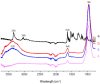
(1) Background: Riparin-A presents several pharmacological activities already elucidated, such as antimicrobial modulator, antileishmania, anxiolytic, anti-inflammatory, antinociceptive, and antioxidant. Even with important bioactive effects, the applicability of Riparin-A is limited due to its low solubility in water, impairing its dissolution in biological fluids. Thus, the objective of this study was to develop a nanohybrid based on Riparin-A and Laponite to obtain a better dissolution profile and evaluate its cytotoxic potential. (2) Methods: The formation of a hybrid system was highlighted by X-ray powder diffraction, infrared spectroscopy, and thermal analysis. Solubility, dissolution, and cytotoxicity studies were performed; (3) Results: An increase in the solubility and aqueous dissolution rate of Riparin-A was observed in the presence of clay. Diffractometric analysis of the hybrid system suggests the amorphization of Riparin-A, and thermal analyses indicated attenuation of decomposition and melting of the Riparin-A after interaction with clay. Furthermore, the nanosystem did not exhibit cytotoxic activity on normal and tumorigenic lines. (4) Conclusions: These results are promising for the development of the Riparin-A/Laponite nanosystem for therapeutic purposes, suggesting an increase in the range of possible routes of administration and bioavailability of this bioactive compound.
Keywords: Laponite; Riparin-A; cytotoxicity; dissolution; nanohybrid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Barbosa Filho J.M., Silva E.C., Bhattacharyya J. Synthesis of Several New Phenylethylamides of Substituted Benzoic Acids. Quim. Nova. 1990;13:332–334.
-
- Nunes G.B.L., Policarpo P.R., Costa L.M., da Silva T.G., Militão G.C.G., Câmara C.A., Barbosa Filho J.M., Gutierrez S.J.C., Islam M.T., de Freitas R.M. In Vitro Antioxidant and Cytotoxic Activity of Some Synthetic Riparin-Derived Compounds. Molecules. 2014;19:4595–4607. doi: 10.3390/molecules19044595. - DOI - PMC - PubMed
-
- Barbosa C.R.d.S., Bezerra A.H., Bezerra S.R., Macêdo N.S., Oliveira-Tintino C.D.d.M., Costa J.G.M.d., Coutinho H.D.M., dos Santos H.S., Cunha F.A.B. da Bioactivities of Isolated and Synthetic Riparins of Aniba Riparia (NEES) MEZ (LAURACEAE): A Brief Review. Phytochem. Lett. 2022;52:149–160. doi: 10.1016/j.phytol.2022.10.009. - DOI
-
- Costa L.M., Macedo E.V., Oliveira F.A.A., Ferreira J.H.L., Gutierrez S.J.C., Peláez W.J., Lima F.C.A., de Siqueira Júnior J.P., Coutinho H.D.M., Kaatz G.W., et al. Inhibition of the NorA Efflux Pump of Staphylococcus Aureus by Synthetic Riparins. J. Appl. Microbiol. 2016;121:1312–1322. doi: 10.1111/jam.13258. - DOI - PubMed
LinkOut - more resources
Full Text Sources

