The Influence of Wet Granulation Parameters on the Compaction Behavior and Tablet Strength of a Hydralazine Powder Mixture
- PMID: 37631362
- PMCID: PMC10458136
- DOI: 10.3390/pharmaceutics15082148
The Influence of Wet Granulation Parameters on the Compaction Behavior and Tablet Strength of a Hydralazine Powder Mixture
Abstract
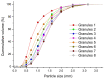
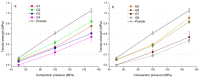
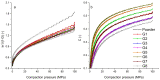
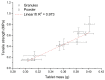
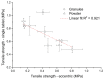
The aim of this paper was to describe the influence of high-shear wet granulation process parameters on tablet tensile strength and compaction behavior of a powder mixture and granules containing hydralazine. The hydralazine powder mixture and eight types of granules were compacted into tablets and evaluated using the Heckel, Kawakita and Adams analyses. The granules were created using two types of granulation liquid (distilled water and aqueous solution of polyvinylpyrrolidone), at different impeller speeds (500 and 700 rpm) and with different wet massing times (without wet massing and for 2 min). Granulation resulted in improved compressibility, reduced dustiness and narrower particle-size distribution. A significant influence of wet massing time on parameters from the Kawakita and Adams analysis was found. Wet massing time had an equally significant effect on tablet tensile strength, regardless of the granulation liquid used. Granules formed with the same wet massing time showed the same trends in tabletability graphs. Tablets created using a single-tablet press (batch compaction) and an eccentric tablet press showed opposite values of tensile strength. Tablets from granules with a higher bulk density showed lower strength during batch compaction and, conversely, higher strength during eccentric tableting.
Keywords: compaction; dynamic image analysis; high-shear granulation; hydralazine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Genedy S., Khames A., Hussein A., Sarhan H. Hydralazine HCl rapidly disintegrating sublingual tablets: Simple dosage form of higher bioavailability and enhanced clinical efficacy for potential rapid control on hypertensive preeclampsia. Drug Des. Devel. Ther. 2018;12:3753–3766. doi: 10.2147/DDDT.S173326. - DOI - PMC - PubMed
-
- Agarwal D.S.P., Ahuja A. Preparation and evaluation of muco-adhesive buccal tablets of hydralazine hydrochloride. Indian J. Pharm. Sci. 1997;59:135–141.
-
- Tam S.W., Sabolinski M.L., Worcel M., Packer M., Cohn J.N. Lack of bioequivalence between different formulations of isosorbide dinitrate and hydralazine and the fixed-dose combination of isosorbide dinitrate/hydralazine: The V-HeFT paradox. Clin. Pharmacokinet. 2007;46:885–895. doi: 10.2165/00003088-200746100-00006. - DOI - PubMed
Grants and funding
- APVV-21-0323/Slovak Research and Development Agency
- APVV-20-0317/Slovak Research and Development Agency
- KEGA 021STU-4/2022/Ministry of Education, Science, Research and Sport of the Slovak Republic
- VEGA 1/0070/22/Ministry of Education, Science, Research and Sport of the Slovak Republic
- 313021BXZ1/European Regional Development Fund
LinkOut - more resources
Full Text Sources

