Wavelet Analysis of Respiratory Muscle sEMG Signals during the Physiological Breakpoint of Static Dry End-Expiratory Breath-Holding in Naive Apneists: A Pilot Study
- PMID: 37631736
- PMCID: PMC10459781
- DOI: 10.3390/s23167200
Wavelet Analysis of Respiratory Muscle sEMG Signals during the Physiological Breakpoint of Static Dry End-Expiratory Breath-Holding in Naive Apneists: A Pilot Study
Abstract
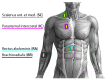
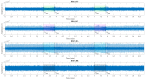
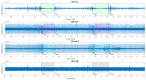
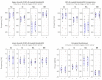
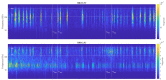
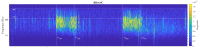
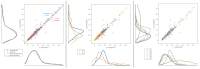
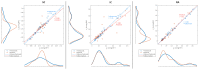
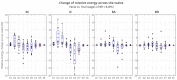
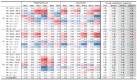
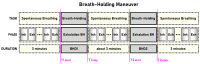
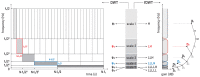
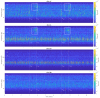
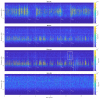
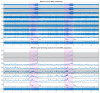
The wavelet spectral characteristics of three respiratory muscle signals (scalenus (SC), parasternal intercostal (IC), and rectus abdominis (RA)) and one locomotor muscle (brachioradialis (BR)) were analyzed in the time-frequency (T-F) domain during voluntary breath-holding (BH), with a focus on the physiological breakpoint that is commonly considered the first involuntary breathing movement (IBM) that signals the end of the easy-going phase of BH. The study was performed for an end-expiratory BH physiological breaking point maneuver on twelve healthy, physically active, naive breath-holders/apneists (six professional athletes; six recreational athletes, and two individuals in the post-COVID-19 period) using surface electromyography (sEMG). We observed individual effects that were dependent on muscle oxygenation and each person's fitness, which were consistent with the mechanism of motor unit (MU) recruitment and the transition of slow-twitch oxidative (type 1) to fast-twitch glycolytic (type 2) muscle fibers. Professional athletes had longer BH durations (BHDs) and strong hypercapnic responses regarding the expiratory RA muscle, which is activated abruptly at higher BHDs in a person-specific range below 250 Hz and is dependent on the BHD. This is in contrast with recreational athletes, who had strong hypoxic responses regarding inspiratory IC muscle, which is activated faster and gradually in the frequency range of 250-450 Hz (independent of the person and BHD). This pilot study preliminarily indicates that it is possible to noninvasively assess the physiological characteristics of skeletal muscles, especially oxygenation, and improve physical fitness tests by determining the T-F features of elevated myoelectric IC and RA activity during BH.
Keywords: breath-holding; heart rate variability; hypercapnia; hypoxia; involuntary breathing movement; multiresolution analysis; muscle fiber subtypes; wavelet analysis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Respiratory muscle activity in voluntary breathing tracking tasks: Implications for the assessment of respiratory motor control.Respir Physiol Neurobiol. 2020 Mar;274:103353. doi: 10.1016/j.resp.2019.103353. Epub 2019 Nov 21. Respir Physiol Neurobiol. 2020. PMID: 31760130
-
Respiratory muscle pressure development during breath holding in apnea divers.Med Sci Sports Exerc. 2013 Jan;45(1):93-101. doi: 10.1249/MSS.0b013e3182690e6a. Med Sci Sports Exerc. 2013. PMID: 22811036
-
Mechanical significance of respiratory muscle activity in humans during halothane anesthesia.Anesthesiology. 1996 Feb;84(2):309-21. doi: 10.1097/00000542-199602000-00008. Anesthesiology. 1996. PMID: 8602661
-
Physiology of static breath holding in elite apneists.Exp Physiol. 2018 May 1;103(5):635-651. doi: 10.1113/EP086269. Exp Physiol. 2018. PMID: 29512224 Review.
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
References
-
- Bain A.R., Ainslie P.N., Barak O.F., Hoiland R.L., Drvis I., Mijacika T., Bailey D.M., Santoro A., DeMasi D.K., Dujic Z., et al. Hypercapnia is essential to reduce the cerebral oxidative metabolism during extreme apnea in humans. J. Cereb. Blood Flow Metab. 2017;37:3231–3242. doi: 10.1177/0271678X16686093. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

