Pustular Eruption following COVID-19 Vaccination: A Narrative Case-Based Review
- PMID: 37631866
- PMCID: PMC10459299
- DOI: 10.3390/vaccines11081298
Pustular Eruption following COVID-19 Vaccination: A Narrative Case-Based Review
Abstract
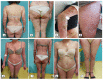
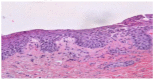
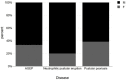
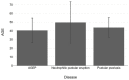
From the beginning of public vaccinations until the relaxation of COVID-19 measures, many case reports, case series and case-control studies have been published indicating cutaneous side effects of COVID-19 vaccination. Post-vaccination pustular eruption was reported as well, with a challenging differential diagnosis between pustular psoriasis, AGEP (acute generalized exanthematous pustulosis) and neutrophil pustular eruptions. We report a case of 56-year-old woman presented with acute generalized pustular flare up culminated 5 days after the second dose of BNT162b2(Pfizer) vaccination. She was diagnosed with pustular psoriasis flare and due to the regulating role of IL-1 in pustular psoriasis and in the cytokine storm observed in cases of COVID-19 postvaccination inflammation; we decided to treat the patient with an IL-1 antagonist, subcutaneous anakinra (100 mg daily) along with acitretin. One week later, after anakinra withdrawal, she presented a pustular psoriasis flare and a 7-day anakinra re-administration led to a satisfactory improvement in the skin lesions. We also reviewed the medical literature and found 28 case reports with pustular eruption after the COVID-19 vaccination. We compared the patients reported, regarding sex, age, number of doses, post-vaccination period and vaccine brand, and compared those results with our patient. Finally, as indicated by our case and other cases with similarly treated pustular eruptions. targeted therapy to this cytokine imbalance such as anakinra (IL-1) antagonist can improve the clinical course of the patient.
Keywords: COVID-19; acute generalized exanthematous pustulosis; neutrophilic pustular eruption; pustular eruption; pustular psoriasis; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

