Maternal and Infant Histo-Blood Group Antigen (HBGA) Profiles and Their Influence on Oral Rotavirus Vaccine (RotarixTM) Immunogenicity among Infants in Zambia
- PMID: 37631871
- PMCID: PMC10458424
- DOI: 10.3390/vaccines11081303
Maternal and Infant Histo-Blood Group Antigen (HBGA) Profiles and Their Influence on Oral Rotavirus Vaccine (RotarixTM) Immunogenicity among Infants in Zambia
Abstract
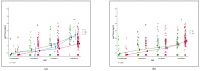
Live-attenuated, oral rotavirus vaccines have significantly reduced rotavirus-associated diarrhoea morbidity and infant mortality. However, vaccine immunogenicity is diminished in low-income countries. We investigated whether maternal and infant intrinsic susceptibility to rotavirus infection via histo-blood group antigen (HBGA) profiles influenced rotavirus (ROTARIX®) vaccine-induced responses in Zambia. We studied 135 mother-infant pairs under a rotavirus vaccine clinical trial, with infants aged 6 to 12 weeks at pre-vaccination up to 12 months old. We determined maternal and infant ABO/H, Lewis, and secretor HBGA phenotypes, and infant FUT2 HBGA genotypes. Vaccine immunogenicity was measured as anti-rotavirus IgA antibody titres. Overall, 34 (31.3%) children were seroconverted at 14 weeks, and no statistically significant difference in seroconversion was observed across the various HBGA profiles in early infant life. We also observed a statistically significant difference in rotavirus-IgA titres across infant HBGA profiles at 12 months, though no statistically significant difference was observed between the study arms. There was no association between maternal HBGA profiles and infant vaccine immunogenicity. Overall, infant HBGAs were associated with RV vaccine immunogenicity at 12 months as opposed to in early infant life. Further investigation into the low efficacy of ROTARIX® and appropriate intervention is key to unlocking the full vaccine benefits for U5 children.
Keywords: Zambia; histo-blood groups; immunogenicity; rotavirus; vaccines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Chilengi R., Rudd C., Bolton C., Guffey B., Masumbu P.K., Stringer J. Successes, Challenges and Lessons Learned in Accelerating Introduction of Rotavirus Immunisation in Zambia. World J. Vaccines. 2015;5:43–53. doi: 10.4236/wjv.2015.51006. - DOI
-
- Church J.A., Parker E.P., Kirkpatrick B.D., Grassly N.C., Prendergast A.J. Interventions to Improve Oral Vaccine Performance in developing countries: A Systematic Review and Meta-analysis Protocol Information. Lancet Infect. Dis. 2017;19:203–214. doi: 10.1016/S1473-3099(18)30602-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

