Next-Generation TB Vaccines: Progress, Challenges, and Prospects
- PMID: 37631874
- PMCID: PMC10457792
- DOI: 10.3390/vaccines11081304
Next-Generation TB Vaccines: Progress, Challenges, and Prospects
Abstract
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is a prevalent global infectious disease and a leading cause of mortality worldwide. Currently, the only available vaccine for TB prevention is Bacillus Calmette-Guérin (BCG). However, BCG demonstrates limited efficacy, particularly in adults. Efforts to develop effective TB vaccines have been ongoing for nearly a century. In this review, we have examined the current obstacles in TB vaccine research and emphasized the significance of understanding the interaction mechanism between MTB and hosts in order to provide new avenues for research and establish a solid foundation for the development of novel vaccines. We have also assessed various TB vaccine candidates, including inactivated vaccines, attenuated live vaccines, subunit vaccines, viral vector vaccines, DNA vaccines, and the emerging mRNA vaccines as well as virus-like particle (VLP)-based vaccines, which are currently in preclinical stages or clinical trials. Furthermore, we have discussed the challenges and opportunities associated with developing different types of TB vaccines and outlined future directions for TB vaccine research, aiming to expedite the development of effective vaccines. This comprehensive review offers a summary of the progress made in the field of novel TB vaccines.
Keywords: Mycobacterium tuberculosis (MTB); clinical trials; deep learning; tuberculosis (TB); vaccines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
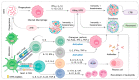
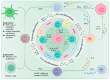


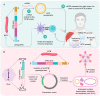
References
-
- WHO . Global Tuberculosis Report 2022. World Health Organization; Geneva, Switzerland: 2022. pp. 1–49.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

