RNA Vaccines: Yeast as a Novel Antigen Vehicle
- PMID: 37631902
- PMCID: PMC10459952
- DOI: 10.3390/vaccines11081334
RNA Vaccines: Yeast as a Novel Antigen Vehicle
Abstract
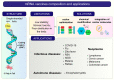
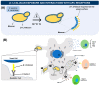
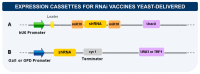
In the last decades, technological advances for RNA manipulation enabled and expanded its application in vaccine development. This approach comprises synthetic single-stranded mRNA molecules that direct the translation of the antigen responsible for activating the desired immune response. The success of RNA vaccines depends on the delivery vehicle. Among the systems, yeasts emerge as a new approach, already employed to deliver protein antigens, with efficacy demonstrated through preclinical and clinical trials. β-glucans and mannans in their walls are responsible for the adjuvant property of this system. Yeast β-glucan capsules, microparticles, and nanoparticles can modulate immune responses and have a high capacity to carry nucleic acids, with bioavailability upon oral immunization and targeting to receptors present in antigen-presenting cells (APCs). In addition, yeasts are suitable vehicles for the protection and specific delivery of therapeutic vaccines based on RNAi. Compared to protein antigens, the use of yeast for DNA or RNA vaccine delivery is less established and has fewer studies, most of them in the preclinical phase. Here, we present an overview of the attributes of yeast or its derivatives for the delivery of RNA-based vaccines, discussing the current challenges and prospects of this promising strategy.
Keywords: antigenic delivery; delivery vehicle; mRNA vaccines; therapeutic vaccines; whole yeast vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
Publication types
Grants and funding
- Facepe/PPSUS APQ-0260-2.02/20/Fundação de Amparo à Ciência e Tecnologia de Pernambuco
- Bolsa produtividade number 308489/2019-5/National Council for Scientific and Technological Development
- CAPES - Epidemias, grant number 88887.507421/ 449 2020-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- CNPq/MCTI/CT-BIOTEC Nº 31/2022, grant number 440315/2022-0/National Council for Scientific and Technological Development
- Programa de Apoio Fixação de Jovens Doutores em Pernambuco APQ-1482-2.02/22/Fundação de Amparo à Ciência e Tecnologia de Pernambuco
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

