Characterisation of a Live-Attenuated Rabies Virus Expressing a Secreted scFv for the Treatment of Rabies
- PMID: 37632016
- PMCID: PMC10458464
- DOI: 10.3390/v15081674
Characterisation of a Live-Attenuated Rabies Virus Expressing a Secreted scFv for the Treatment of Rabies
Abstract
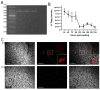
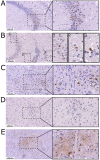
Rabies virus (RABV) causes possibly the oldest disease and is responsible for an estimated >59,000 human fatalities/year. Post exposure prophylaxis (PEP), the administration of vaccine and rabies immunoglobulin, is a highly effective tool which is frequently unavailable in RABV endemic areas. Furthermore, due to the constraints of the blood-brain barrier, current PEP regimes are ineffective after the onset of clinical symptoms which invariably result in death. To circumvent this barrier, a live-attenuated recombinant RABV expressing a highly RABV-neutralising scFv antibody (62-71-3) linked to the fluorescent marker mCherry was designed. Once rescued, the resulting construct (named RABV-62scFv) was grown to high titres, its growth and cellular dissemination kinetics characterised, and the functionality of the recombinant 62-71-3 scFv assessed. Encouraging scFv production and subsequent virus neutralisation results demonstrate the potential for development of a therapeutic live-attenuated virus-based post-infection treatment (PIT) for RABV infection.
Keywords: PEP; antibody; lyssavirus; rabies; rabies treatment; scFv; virus attenuation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Borca M.V., Ramirez-Medina E., Silva E., Vuono E., Rai A., Pruitt S., Holinka L.G., Velazquez-Salinas L., Zhu J., Gladue D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020;94:10–1128. doi: 10.1128/JVI.02017-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

