Laboratory Based Surveillance of HIV-1 Acquired Drug Resistance in Cameroon: Implications for Use of Tenofovir-Lamivudine-Dolutegravir (TLD) as Second- or Third-Line Regimens
- PMID: 37632026
- PMCID: PMC10459610
- DOI: 10.3390/v15081683
Laboratory Based Surveillance of HIV-1 Acquired Drug Resistance in Cameroon: Implications for Use of Tenofovir-Lamivudine-Dolutegravir (TLD) as Second- or Third-Line Regimens
Abstract
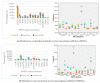
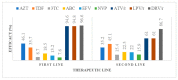
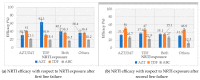
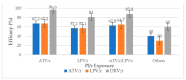
Increased HIV drug resistance (HIVDR) with antiretroviral therapy (ART) rollout may jeopardize therapeutic options, especially in this era of transition to fixed-dose tenofovir-lamivudine-dolutegravir (TLD). We studied acquired HIVDR (ADR) patterns and describe potentially active drugs after first- and second-line failure in resource-limited settings (RLS) like Cameroon. A laboratory-based study with 759 patients (≥15 years) experiencing virological failure was carried out at the Chantal Biya International Reference Centre (CIRCB), Yaoundé, Cameroon. Socio-demographic, therapeutic and immunovirological data from patient records were analysed according to HIV-1 genotypic profiles. Median (IQR) ART-duration was 63 (50-308) months. Median CD4 and viremia were 153 (IQR:50-308) cells/mm3 and 138,666 (IQR:28,979-533,066) copies/mL, respectively. Overall ADR was high (93.4% first-line; 92.9%-second-line). TDF, potentially active in 35.7% of participants after first-line and 45.1% after second-line, suggested sub-optimal TLD-efficacy in second-line (64.3%) and third-line (54.9%). All PI/r preserved high efficacy after first-line failure while only DRV/r preserved high-level efficacy (87.9%) after second-line failure. In this resource-limited setting (RLS), ADR is high in ART-failing patients. PI/r strategies remain potent backbones for second-line ART, while only DRV/r remains very potent despite second-line failure. Though TLD use would be preferable, blind use for second- and third-line regimens may be sub-optimal (functional monotherapy with dolutegravir) with high risk of further failure, thus suggesting strategies for selective ART switch to TLD in failing patients in RLS.
Keywords: TLD; acquired drug resistance; first-line failure; laboratory-based surveillance; second-line failure; tenofovir-lamivudine-dolutegravir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Viral suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: a prospective cohort study.Lancet HIV. 2022 Aug;9(8):e544-e553. doi: 10.1016/S2352-3018(22)00136-9. Lancet HIV. 2022. PMID: 35905753
-
Viral suppression in the era of transition to dolutegravir-based therapy in Cameroon: Children at high risk of virological failure due to the lowly transition in pediatrics.Medicine (Baltimore). 2023 May 19;102(20):e33737. doi: 10.1097/MD.0000000000033737. Medicine (Baltimore). 2023. PMID: 37335723 Free PMC article.
-
Dolutegravir-Based Regimen Ensures High Virological Success despite Prior Exposure to Efavirenz-Based First-LINE ART in Cameroon: An Evidence of a Successful Transition Model.Viruses. 2022 Dec 21;15(1):18. doi: 10.3390/v15010018. Viruses. 2022. PMID: 36680058 Free PMC article.
-
HIV-1 pretreatment and acquired antiretroviral drug resistance before tenofovir/ /lamivudine /dolutegravir (TLD) roll-out in Mozambique.BMC Infect Dis. 2024 Jul 29;24(1):748. doi: 10.1186/s12879-024-09579-4. BMC Infect Dis. 2024. PMID: 39075381 Free PMC article.
-
Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection.Drugs. 2015 Apr;75(5):503-14. doi: 10.1007/s40265-015-0361-6. Drugs. 2015. PMID: 25698454 Review.
Cited by
-
Predictive Efficacy of Dual Therapies Combining Integrase Strand Transfer Inhibitors with Second-Generation Non-Nucleoside Reverse Transcriptase Inhibitors Following HIV-1 Treatment Failure in Cameroon: Implications for the Use of a Long-Acting Therapeutic Strategy in Low- and Middle-Income Countries.Viruses. 2024 Nov 29;16(12):1853. doi: 10.3390/v16121853. Viruses. 2024. PMID: 39772163 Free PMC article.
-
Retrospective Study on Genetic Diversity and Drug Resistance among People Living with HIV at an AIDS Clinic in Beijing.Pharmaceuticals (Basel). 2024 Jan 16;17(1):115. doi: 10.3390/ph17010115. Pharmaceuticals (Basel). 2024. PMID: 38256948 Free PMC article.
-
Longer-term virologic outcomes on tenofovir-lamivudine-dolutegravir in second-line ART.South Afr J HIV Med. 2025 Apr 30;26(1):1677. doi: 10.4102/sajhivmed.v26i1.1677. eCollection 2025. South Afr J HIV Med. 2025. PMID: 40356938 Free PMC article.
-
Effectiveness of dolutegravir-based treatment among HIV/AIDS patients in Nkembo Outpatient Treatment Center, Gabon.AIDS Res Ther. 2025 May 3;22(1):50. doi: 10.1186/s12981-025-00744-6. AIDS Res Ther. 2025. PMID: 40319303 Free PMC article.
References
-
- Global HIV & AIDS Statistics—Fact Sheet. [(accessed on 9 March 2023)]. Available online: https://www.unaids.org/en/resources/fact-sheet.
-
- HIV Data and Statistics. [(accessed on 5 September 2022)]. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/s....
-
- TimesNews2 HIV Prevalence Rate in Cameroon Drops to 2.7%. TimesNews2. 2019. [(accessed on 21 June 2020)]. Available online: http://timesnews2.info/hiv-prevalence-rate-in-cameroon-drops-to-2-7/
-
- Sidibe M. UNAIDS DATA 2017. 2017. [(accessed on 2 June 2018)]. p. 54. Available online: http://aidsinfo.unaids.org.
-
- The World Bank Group The World Bank Data—Antiretroviral Therapy Coverage (% of people living with HIV) 2017. [(accessed on 16 December 2017)]. Available online: https://data.worldbank.org/indicator/SH.HIV.ARTC.ZS.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

