Compartmentalized Regulation of Pulmonary and Systemic Inflammation in Critical COVID-19 Patients
- PMID: 37632046
- PMCID: PMC10458965
- DOI: 10.3390/v15081704
Compartmentalized Regulation of Pulmonary and Systemic Inflammation in Critical COVID-19 Patients
Abstract
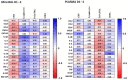
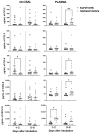
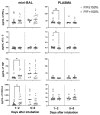
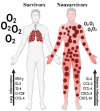
Critical COVID-19 has been associated with altered patterns of cytokines. Distinct inflammatory processes in systemic and pulmonary sites have been reported, but studies comparing these two sites are still scarce. We aimed to evaluate the profile of pulmonary and systemic cytokines and chemokines in critically ill COVID-19 patients. Levels of cytokines and chemokines were measured in plasma samples and minibronchoalveolar lavage of critical COVID-19 patients within 48 h and 5-8 days after intubation. Distinct inflammatory processes were observed in the lungs and blood, which were regulated separately. Survivor patients showed higher lung cytokine levels including IFN-γ, IL-2, IL-4, G-CSF, and CCL4, while nonsurvivors displayed higher levels in the blood, which included IL-6, CXCL8, CXCL10, CCL2, and CCL4. Furthermore, our findings indicate that high TNF and CXCL8 levels in the mini-BAL were associated with better lung oxygen exchange capacity, whereas high levels of IFN-γ in plasma were associated with worse lung function, as measured using the PaO2/FiO2 ratio. These results suggest that a robust and localized inflammatory response in the lungs is protective and associated with survival, whereas a systemic inflammatory response is detrimental and associated with mortality in critical COVID-19.
Keywords: COVID-19; SARS-CoV-2; blood; chemokines; cytokines; inflammation; lung.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jonhs Hopkins University COVID-19 Dashboard. [(accessed on 3 March 2023)]. Available online: https://coronavirus.jhu.edu/map.html.
-
- Galani I.-E., Rovina N., Lampropoulou V., Triantafyllia V., Manioudaki M., Pavlos E., Koukaki E., Fragkou P.C., Panou V., Rapti V., et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021;22:32–40. doi: 10.1038/s41590-020-00840-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

