Feline Injection-Site Sarcoma and Other Adverse Reactions to Vaccination in Cats
- PMID: 37632050
- PMCID: PMC10459272
- DOI: 10.3390/v15081708
Feline Injection-Site Sarcoma and Other Adverse Reactions to Vaccination in Cats
Abstract
Vaccine-associated adverse events (VAAEs), including feline injection-site sarcomas (FISSs), occur only rarely but can be severe. Understanding potential VAAEs is an important part of informed owner consent for vaccination. In this review, the European Advisory Board on Cat Diseases (ABCD), a scientifically independent board of feline medicine experts, presents the current knowledge on VAAEs in cats, summarizing the literature and filling the gaps where scientific studies are missing with expert opinion to assist veterinarians in adopting the best vaccination practice. VAAEs are caused by an aberrant innate or adaptive immune reaction, excessive local reactions at the inoculation site, an error in administration, or failure in the manufacturing process. FISS, the most severe VAAE, can develop after vaccinations or injection of other substances. Although the most widely accepted hypothesis is that chronic inflammation triggers malignant transformation, the pathogenesis of FISS is not yet fully understood. No injectable vaccine is risk-free, and therefore, vaccination should be performed as often as necessary, but as infrequently as possible. Vaccines should be brought to room temperature prior to administration and injected at sites in which FISS surgery would likely be curative; the interscapular region should be avoided. Post-vaccinal monitoring is essential.
Keywords: FISS; VAAE; adverse event; anaphylaxis; hypersensitivity reaction; immunization; side effect; vaccine.
Conflict of interest statement
The authors are members of the European Advisory Board on Cat Diseases (ABCD), a scientifically independent board of experts in feline medicine. Some individual authors have been consultants for, or have received research funding and honoraria from, animal health companies and other organizations, but all authors declare no conflict of interest for the writing of these independent guidelines. The ABCD sponsors had no role in the writing, nor in the decision to publish this review.
Figures

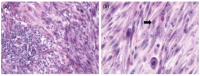
References
-
- European Advisory Board on Cat Diseases Guideline for Adverse Reactions to Vaccination. [(accessed on 13 January 2023)]. Available online: http://www.abcdcatsvets.org/adverse-reactions-to-vaccination/
-
- Hosie M.J., Addie D., Belák S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Lloret A., Lutz H., et al. Matrix vaccination guidelines: ABCD recommendations for indoor/outdoor cats, rescue shelter cats and breeding catteries. J. Feline Med. Surg. 2013;15:540–544. doi: 10.1177/1098612X13489209. - DOI - PMC - PubMed
-
- Hartmann K., Day M.J., Thiry E., Lloret A., Frymus T., Addie D., Boucraut-Baralon C., Egberink H., Gruffydd-Jones T., Horzinek M.C., et al. Feline injection-site sarcoma: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015;17:606–613. doi: 10.1177/1098612X15588451. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

