Evaluation of Nafamostat as Chemoprophylaxis for SARS-CoV-2 Infection in Hamsters
- PMID: 37632086
- PMCID: PMC10458615
- DOI: 10.3390/v15081744
Evaluation of Nafamostat as Chemoprophylaxis for SARS-CoV-2 Infection in Hamsters
Abstract
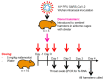
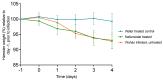
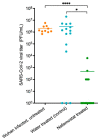
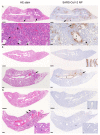
The successful development of a chemoprophylaxis against SARS-CoV-2 could provide a tool for infection prevention that is implementable alongside vaccination programmes. Nafamostat is a serine protease inhibitor that inhibits SARS-CoV-2 entry in vitro, but it has not been characterised for chemoprophylaxis in animal models. Clinically, nafamostat is limited to intravenous delivery and has an extremely short plasma half-life. This study sought to determine whether intranasal dosing of nafamostat at 5 mg/kg twice daily was able to prevent the airborne transmission of SARS-CoV-2 from infected to uninfected Syrian Golden hamsters. SARS-CoV-2 RNA was detectable in the throat swabs of the water-treated control group 4 days after cohabitation with a SARS-CoV-2 inoculated hamster. However, throat swabs from the intranasal nafamostat-treated hamsters remained SARS-CoV-2 RNA negative for the full 4 days of cohabitation. Significantly lower SARS-CoV-2 RNA concentrations were seen in the nasal turbinates of the nafamostat-treated group compared to the control (p = 0.001). A plaque assay quantified a significantly lower concentration of infectious SARS-CoV-2 in the lungs of the nafamostat-treated group compared to the control (p = 0.035). When taken collectively with the pathological changes observed in the lungs and nasal mucosa, these data are strongly supportive of the utility of intranasally delivered nafamostat for the prevention of SARS-CoV-2 infection.
Keywords: SARS-CoV-2; chemoprophylaxis; nafamostat.
Conflict of interest statement
A.O. and S.R. are Directors of Tandem Nano Ltd. M.N., J.H., A.O. and S.R. are co-inventors of patents relating to drug delivery. A.O. has received research funding from ViiV, Merck, Janssen and consultancy from Gilead, ViiV and Merck not related to the current paper. S.R. has received research funding from ViiV and AstraZeneca and consultancy from Gilead not related to the current paper. J.P.S. has received research funding from ENA respiratory, and consultancy from Byotrol Technologies not related to current paper. No other conflicts are declared by the authors.
Figures


References
-
- Kuzmina A., Khalaila Y., Voloshin O., Keren-Naus A., Boehm-Cohen L., Raviv Y., Avni Y., Rosenberg R., Taube R. SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microb. 2021;29:522–528.e2. doi: 10.1016/j.chom.2021.03.008. - DOI - PMC - PubMed
-
- Li H., van der Linden W.A., Verdoes M., Florea B.I., McAllister F.E., Govindaswamy K., Elias J.E., Bhanot P., Overkleeft H.S., Bogyo M. Assessing subunit dependency of the Plasmodium proteasome using small molecule inhibitors and active site probes. ACS Chem. Biol. 2014;9:1869–1876. doi: 10.1021/cb5001263. - DOI - PMC - PubMed
-
- Christ F., Shaw S., Demeulemeester J., Desimmie B.A., Marchand A., Butler S., Smets W., Chaltin P., Westby M., Debyser Z., et al. Small-molecule inhibitors of the LEDGF/p75 binding site of integrase block HIV replication and modulate integrase multimerization. Antimicrob. Agents Chemother. 2012;56:4365–4374. doi: 10.1128/AAC.00717-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

