Detection of High-Risk Paraneoplastic Antibodies against TRIM9 and TRIM67 Proteins
- PMID: 37632288
- PMCID: PMC10842626
- DOI: 10.1002/ana.26776
Detection of High-Risk Paraneoplastic Antibodies against TRIM9 and TRIM67 Proteins
Abstract
Objective: Co-occurring anti-tripartite motif-containing protein 9 and 67 autoantibodies (TRIM9/67-IgG) have been reported in only a very few cases of paraneoplastic cerebellar syndrome. The value of these biomarkers and the most sensitive methods of TRIM9/67-IgG detection are not known.
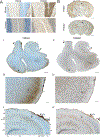
Methods: We performed a retrospective, multicenter study to evaluate the cerebrospinal fluid and serum of candidate TRIM9/67-IgG cases by tissue-based immunofluorescence, peptide phage display immunoprecipitation sequencing, overexpression cell-based assay (CBA), and immunoblot. Cases in which TRIM9/67-IgG was detected by at least 2 assays were considered TRIM9/67-IgG positive.
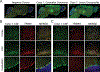
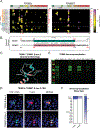
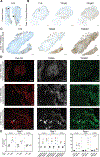
Results: Among these cases (n = 13), CBA was the most sensitive (100%) and revealed that all cases had TRIM9 and TRIM67 autoantibodies. Of TRIM9/67-IgG cases with available clinical history, a subacute cerebellar syndrome was the most common presentation (n = 7/10), followed by encephalitis (n = 3/10). Of these 10 patients, 70% had comorbid cancer (7/10), 85% of whom (n = 6/7) had confirmed metastatic disease. All evaluable cancer biopsies expressed TRIM9 protein (n = 5/5), whose expression was elevated in the cancerous regions of the tissue in 4 of 5 cases.
Interpretation: TRIM9/67-IgG is a rare but likely high-risk paraneoplastic biomarker for which CBA appears to be the most sensitive diagnostic assay. ANN NEUROL 2023;94:1086-1101.
© 2023 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
ALP reports grant and research support from Genentech; consulting fees from Alexion, Genentech/Roche, UCB, and EMD Sorono. MRW has received research support from Roche/Genentech and Novartis as well as speaking honoraria from Novartis, Takeda, and Genentech, companies that make therapeutics that could be relevant to paraneoplastic disease. SJP and AZ work as consultants in the Mayo Clinic Neuroimmunology Laboratory clinical service that commercially offers neural autoantibody testing, but revenue accrued does not contribute to salary, research support, or personal income for any of the authors. JH and DLD have filed for a patent (PCT/EP/2019/061280) to protect biological tests to detect anti-TRIM9/67 autoantibodies in patients. All other authors have nothing to report.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH122471/MH/NIMH NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- R25MH060482/MH/NIMH NIH HHS/United States
- S10 OD028511/OD/NIH HHS/United States
- P30 CA082103/CA/NCI NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- R01 AG031189/AG/NIA NIH HHS/United States
- K08 NS096117/NS/NINDS NIH HHS/United States
- S10 OD017993/OD/NIH HHS/United States
- K08NS096117/NS/NINDS NIH HHS/United States
- R25 MH060482/MH/NIMH NIH HHS/United States
- R01 GM129325/GM/NIGMS NIH HHS/United States
- P30CA082103/CA/NCI NIH HHS/United States
- R01MH122471/MH/NIMH NIH HHS/United States
- R01 AG062562/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

