Outpatient Worsening Among Patients With Mildly Reduced and Preserved Ejection Fraction Heart Failure in the DELIVER Trial
- PMID: 37632455
- PMCID: PMC10664793
- DOI: 10.1161/CIRCULATIONAHA.123.066506
Outpatient Worsening Among Patients With Mildly Reduced and Preserved Ejection Fraction Heart Failure in the DELIVER Trial
Abstract
Background: Hospitalization is recognized as a sentinel event in the disease trajectory of patients with heart failure (HF), but not all patients experiencing clinical decompensation are ultimately hospitalized. Outpatient intensification of diuretics is common in response to symptoms of worsening HF, yet its prognostic and clinical relevance, specifically for patients with HF with mildly reduced or preserved ejection fraction, is uncertain.
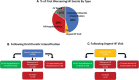
Methods: In this prespecified analysis of the DELIVER trial (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure), we assessed the association between various nonfatal worsening HF events (those requiring hospitalization, urgent outpatient visits requiring intravenous HF therapies, and outpatient oral diuretic intensification) and rates of subsequent mortality. We further examined the treatment effect of dapagliflozin on an expanded composite end point of cardiovascular death, HF hospitalization, urgent HF visit, or outpatient oral diuretic intensification.
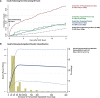
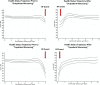
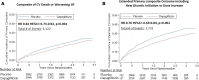
Results: In DELIVER, 4532 (72%) patients experienced no worsening HF event, whereas 789 (13%) had outpatient oral diuretic intensification, 86 (1%) required an urgent HF visit, 585 (9%) had an HF hospitalization, and 271 (4%) died of cardiovascular causes as a first presentation. Patients with a first presentation manifesting as outpatient oral diuretic intensification experienced rates of subsequent mortality that were higher (10 [8-12] per 100 patient-years) than those without a worsening HF event (4 [3-4] per 100 patient-years) but similar to rates of subsequent death after an urgent HF visit (10 [6-18] per 100 patient-years). Patients with an HF hospitalization as a first presentation of worsening HF had the highest rates of subsequent death (35 [31-40] per 100 patient-years). The addition of outpatient diuretic intensification to the adjudicated DELIVER primary end point (cardiovascular death, HF hospitalization, or urgent HF visit) increased the overall number of patients experiencing an event from 1122 to 1731 (a 54% increase). Dapagliflozin reduced the need for outpatient diuretic intensification alone (hazard ratio, 0.72 [95% CI, 0.64-0.82]) and when analyzed as a part of an expanded composite end point of worsening HF or cardiovascular death (hazard ratio, 0.76 [95% CI, 0.69-0.84]).
Conclusions: In patients with HF with mildly reduced or preserved ejection fraction, worsening HF requiring oral diuretic intensification in ambulatory care was frequent, adversely prognostic, and significantly reduced by dapagliflozin.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03619213.
Keywords: disease progression; heart failure; heart failure, diastolic; heart failure, systolic; hospitalization; sodium-glucose transporter 2 inhibitor.
Conflict of interest statement
Figures
Comment in
-
Expanding the Definition of Worsening Heart Failure and Recognizing the Importance of Outpatient Escalation of Oral Diuretics.Circulation. 2023 Nov 28;148(22):1746-1749. doi: 10.1161/CIRCULATIONAHA.123.066915. Epub 2023 Nov 27. Circulation. 2023. PMID: 38011247 No abstract available.
References
-
- Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. ; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 - PubMed
-
- Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, et al. ; Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906 - PubMed
-
- Greene SJ, Bauersachs J, Brugts JJ, Ezekowitz JA, Lam CSP, Lund LH, Ponikowski P, Voors AA, Zannad F, Zieroth S, et al. Worsening heart failure: nomenclature, epidemiology, and future directions: JACC review topic of the week. J Am Coll Cardiol. 2023;81:413–424. doi: 10.1016/j.jacc.2022.11.023 - PubMed
-
- Docherty KF, Jhund PS, Anand I, Bengtsson O, Böhm M, de Boer RA, DeMets DL, Desai AS, Drozdz J, Howlett J, et al. Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA-HF. Circulation. 2020;142:1623–1632. doi: 10.1161/CIRCULATIONAHA.120.047480 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

