Impact of maternally derived meiotic aneuploidies on early embryonic development in vitro
- PMID: 37632639
- PMCID: PMC10643722
- DOI: 10.1007/s10815-023-02922-9
Impact of maternally derived meiotic aneuploidies on early embryonic development in vitro
Erratum in
-
Correction to: Impact of maternally derived meiotic aneuploidies on early embryonic development in vitro.J Assist Reprod Genet. 2024 Oct;41(10):2851. doi: 10.1007/s10815-024-03233-3. J Assist Reprod Genet. 2024. PMID: 39190229 Free PMC article. No abstract available.
Abstract
Purpose: To assess early embryonic developmental potential of embryos affected by maternally inherited meiotic aneuploidies.
Methods: This observational, descriptive study includes 930 oocytes from 151 patients which were retrospectively analyzed by combining the morphological assessment with the genetic results from polar body diagnosis.
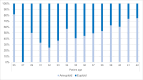
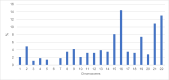
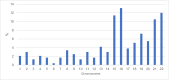
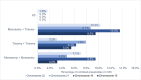
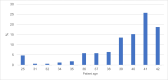
Results: Of 930 oocytes examined, 566 (60.9%) were tested aneuploid. Developmental potential until cleavage stage was not affected by trisomies or monosomies (69.6% vs. 77.1%, p = 0.75). However, trisomies significantly more often resulted in top quality cleavage stage embryos compared to monosomies (20% vs. 17.6%, p = < 0.01). Top quality blastocysts were more likely to be euploid than aneuploid (52.4% vs. 47.6%, p = 0.032). Additionally, significantly more aneuploid embryos resulted in developmental arrest compared to euploid embryos (15.3% vs. 6.7%, p = 0.003). Overall, there was no significant difference in the frequency of trisomies and monosomies in blastocyst stage embryos. (28.3% vs. 28.2%; p = 0.81). In contrast to earlier developmental stages, distribution of trisomies and monosomies did not differ in top quality blastocysts (8.3% vs. 5.3%, p = 0.32). However, certain chromosomal abnormalities showed a higher potential to develop into a top-rated blastocyst. These included monosomies 2, 5, 8, 10, 16, 17, 20, 21, and 22 and trisomies 2, 4, 5, 8, 9, 10, 11, 12, 13, 16, 17, 18 and 20.
Conclusion: Meiotically induced maternal aneuploidies have different effects on early embryonic development. While no difference in developmental potential between monosomies and trisomies could be observed in blastocysts, cleavage stage quality was significantly affected by chromosomal aneuploidies.
Keywords: Aneuploidies; Embryo quality; IVF; PGT-A; Polar bodies.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
None.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

