Continuous Subcutaneous Foslevodopa/Foscarbidopa in Parkinson's Disease: Safety and Efficacy Results From a 12-Month, Single-Arm, Open-Label, Phase 3 Study
- PMID: 37632656
- PMCID: PMC10630297
- DOI: 10.1007/s40120-023-00533-1
Continuous Subcutaneous Foslevodopa/Foscarbidopa in Parkinson's Disease: Safety and Efficacy Results From a 12-Month, Single-Arm, Open-Label, Phase 3 Study
Erratum in
-
Correction: Continuous Subcutaneous Foslevodopa/Foscarbidopa in Parkinson's Disease: Safety and Efficacy Results From a 12-Month, Single-Arm, Open-Label, Phase 3 Study.Neurol Ther. 2023 Dec;12(6):1959-1960. doi: 10.1007/s40120-023-00554-w. Neurol Ther. 2023. PMID: 37817017 Free PMC article. No abstract available.
Abstract
Introduction: Foslevodopa/foscarbidopa, a soluble formulation of levodopa/carbidopa (LD/CD) prodrugs for the treatment of Parkinson's disease (PD), is administered as a 24-hour/day continuous subcutaneous infusion (CSCI) with a single infusion site. The efficacy and safety of foslevodopa/foscarbidopa versus oral immediate-release LD/CD was previously demonstrated in patients with PD in a 12-week, randomized, double-blind, phase 3 trial (NCT04380142). We report the results of a separate 52-week, open-label, phase 3 registrational trial (NCT03781167) that evaluated the safety/tolerability and efficacy of 24-hour/day foslevodopa/foscarbidopa CSCI in patients with advanced PD.
Methods: Male and female patients with levodopa-responsive PD and ≥ 2.5 hours of "Off" time/day received 24-hour/day foslevodopa/foscarbidopa CSCI at individually optimized therapeutic doses (approximately 700-4250 mg of LD per 24 hours) for 52 weeks. The primary endpoint was safety/tolerability. Secondary endpoints included changes from baseline in normalized "Off" and "On" time, percentage of patients reporting morning akinesia, Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS), Parkinson's Disease Sleep Scale-2 (PDSS-2), 39-item Parkinson's Disease Questionnaire (PDQ-39), and EuroQol 5-dimension questionnaire (EQ-5D-5L).
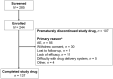
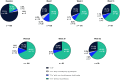
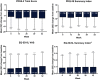
Results: Of 244 enrolled patients, 107 discontinued, and 137 completed treatment. Infusion site events were the most common adverse events (AEs). AEs were mostly nonserious (25.8% of patients reported serious AEs) and mild/moderate in severity. At week 52, "On" time without troublesome dyskinesia and "Off" time were improved from baseline (mean [standard deviation (SD)] change in normalized "On" time without troublesome dyskinesia, 3.8 [3.3] hours; normalized "Off" time, -3.5 [3.1] hours). The percentage of patients experiencing morning akinesia dropped from 77.7% at baseline to 27.8% at week 52. Sleep quality (PDSS-2) and quality of life (PDQ-39 and EQ-5D-5L) also improved.
Conclusion: Foslevodopa/foscarbidopa has the potential to provide a safe and efficacious, individualized, 24-hour/day, nonsurgical alternative for patients with PD.
Trial registration number: ClinicalTrials.gov identifier NCT03781167.
Keywords: Advanced Parkinson’s disease; Foslevodopa/foscarbidopa; Levodopa/carbidopa prodrugs; Motor fluctuations; Subcutaneous infusion.
© 2023. The Author(s).
Conflict of interest statement
Jason Aldred is a study investigator for AbbVie, AC Immune, Annovis, Aptinyx, AstraZeneca, Atara, Athira, Biogen, BioVie, Boston Scientific, Celgene, Cerevance, Cerevel, Denali, EIP, Eli Lilly, Impax, Inhibikase, IRL Therapeutics, Merz, Neuraly, Neurocrine, Neuoderm, Novartis, PD Gene/PSG, Praxis, Revance, Roche/Genentech, Sage, Sanofi/Genzyme, Scion NeuroStim, Takeda, Theravance, Triplet/HSG, and UCB. He has received honorarium from AbbVie, Allergan, Boston Scientific Teva, US World Meds, Medtronic, and Abbot. Eric Freire-Alvarez has received advisory, consulting, and lecture fees from AbbVie, Almirall, Bial, Eisai, UCB Pharma, Teva, and Zambon. He is an investigator on AbbVie studies. Alexander V. Amelin has received advisory, consulting, and lecture fees from AbbVie, Pfizer, Novartis, Lundbeck, UCB Pharma, Teva, Viatris, and Stada. He is an investigator on AbbVie studies. Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Boehringer Ingelheim, Ever Pharma, General Electric, Britannia, AbbVie, Kyowa Kirin, Zambon, Bial, Theravance Biopharma, Jazz Pharmaceuticals, Roche, and Medscape. He receives research support from Bial, Lundbeck, Roche, Angelini Pharmaceuticals, Horizon 2020 Grant 825785, Horizon 2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation, and Movement Disorder Society for NMS Scale validation. He serves as a consultant for Boehringer Ingelheim for legal cases on pathologic gambling. He owns Patent WO2015110261-A1 and owns shares from PD Neurotechnology Limited. Bruno Bergmans has a clinical practice at AZ St-Jan Brugge-Oostende AV in Bruges and is an academic consultant at Ghent University Hospital, Ghent. He has served as an advisory board member for Allergan, Merz, and UCB, and has served as a speaker for Zambon and AbbVie. He is the principal investigator for the subcutaneous levodopa study in Bruges. Filip Bergquist has received financial compensation for lectures and advisory services as well as in-kind donations of PKG reports for clinical studies from GKC, and honorarium for an advisory board from AbbVie. He is a stock option holder of Dizlin Pharmaceuticals AB. Manon Bouchard has received honoraria for consultancy, lectures, and advisory boards from AbbVie, Allergan, Merz, Ipsen, Lilly, Lundbeck, Novartis, Paladin, Sunovion, and UCB. She is also a consultant for Société Parkinson Québec. She has received research support from AbbVie, ES-therapeutics, and Pfizer, and royalties from UptoDate. Kumar Budur is a former employee of AbbVie Inc., and is currently employed by Harmony Biosciences. Camille Carroll has received honoraria for consultancy services from AbbVie, Bial, Britannia, GKC, GSK, Kyowa Kirin, Lundbeck, Medscape, and Scient. She has received service grants from AbbVie and Bial, and research grants from Parkinson’s UK, Cure Parkinson’s, National Institute for Health Research, and the Edmond J. Safra Foundation. She is an employee of Newcastle University, Translational and Clinical Research Institute, University of Plymouth, University Hospitals Plymouth National Health Service Trust, and National Institute of Health and Care Research. K. Ray Chaudhuri is a study investigator and has served as an advisory board member for AbbVie, UCB, GKC, Bial, Cynapsus, Lobsor, Stada, Medtronic, Zambon, Profile, Sunovion, Roche, Therevance, Scion, Britannia, Acadia, and 4D. He received honoraria for lectures from AbbVie, Britannia, UCB, Zambon, Novartis, Boehringer Ingelheim, Bial, Kyowa Kirin, and SK Pharma. He has received grants (investigator initiated) from Britannia Pharmaceuticals, AbbVie, UCB, GKC, and Bial, and academic grants from EU, IMI EU, Horizon 2020, Parkinson’s UK, NIHR, PDNMG, EU (Horizon 2020), Kirby Laing Foundation, NPF, MRC, and Wellcome Trust. He receives royalties from Oxford University Press and holds intellectual property rights for the King’s Parkinson’s Pain Scale and Parkinson’s Disease Sleep Scale. Susan R. Criswell is a former employee of Washington University in St. Louis, St. Louis, MO, USA, and is currently employed by Barrow Neurological Institute, Phoenix, AZ, USA. She reports being site principal investigator for clinical trials with AbbVie, Impax, and the NIH. She receives grant funding from the NIH including grants R01 ES026891, R01ES029524, R01 OH011661, U19 AG071754-01, and R01ES021488. Erik H. Danielsen has received lecture fees from AbbVie, UCB, Nordic Infucare, Allergan, Ibsen, and Merz. Florin Gandor has served as an advisory board member for AbbVie and has received honoraria from AbbVie, Bial Pharma, Merz, and Stada. Thomas E. Kimber has received honoraria from Stada, AbbVie, UCB Pharma, Seqiris, and Teva. Hideki Mochizuki served as a consultant, lecturer, and/or scientific advisor for IQVIA, AbbVie, Insightec, Eisai Co., FP Pharmaceutical Corporation, Otsuka Pharmaceutical, Ono Pharmaceutical Corporation, Kyowa Kirin, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Nihon Medi-Physics, and Novartis Pharma K.K. He has received scholarship grants from Kyowa Kirin, Mochida Pharmaceutical, and Japan Blood Products Organization. David G. Standaert is a member of the faculty of the University of Alabama at Birmingham and is supported by endowment and University funds. He is an investigator in studies funded by AbbVie Inc., the American Parkinson Disease Association, the Michael J. Fox Foundation for Parkinson Research, Alabama Department of Commerce, Alabama Innovation Fund, the Department of Defense, and NIH grants P50NS108675, R25NS079188, and T32NS095775. He has a clinical practice and is compensated for these activities through the University of Alabama Health Services Foundation. In addition, since January 1, 2021, he has served as a consultant for or received honoraria from AbbVie Inc., Sutter Health, Curium Pharma, Appello, Theravance, Sanofi-Aventis, Alnylam Pharmaceuticals, Coave Therapeutics, BlueRock Therapeutics and F. Hoffman-La Roche. Victor S. C. Fung receives a salary from NSW Health. He has received unrestricted research grants from the Michael J Fox Foundation, AbbVie, and Merz, and has been on advisory boards for AbbVie, Allergan, Ipsen, Merz, Praxis, Seqirus, Stada, Teva, and UCB. He receives royalties from Health Press. Jia Jia, Weining Z. Robieson, Amy M. Spiegel, Saritha Talapala, and Maurizio F. Facheris are employees of AbbVie Inc. and may hold AbbVie stock and/or stock options.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

