Toxic effects of mutant huntingtin in axons are mediated by its proline-rich domain
- PMID: 37633260
- PMCID: PMC11146425
- DOI: 10.1093/brain/awad280
Toxic effects of mutant huntingtin in axons are mediated by its proline-rich domain
Erratum in
-
Correction to: Toxic effects of mutant huntingtin in axons are mediated by its proline-rich domain.Brain. 2025 Sep 3;148(9):e85. doi: 10.1093/brain/awaf189. Brain. 2025. PMID: 40439940 Free PMC article. No abstract available.
Abstract
Huntington's disease results from expansion of a polyglutamine tract (polyQ) in mutant huntingtin (mHTT) protein, but mechanisms underlying polyQ expansion-mediated toxic gain-of-mHTT function remain elusive. Here, deletion and antibody-based experiments revealed that a proline-rich domain (PRD) adjacent to the polyQ tract is necessary for mHTT to inhibit fast axonal transport and promote axonal pathology in cultured mammalian neurons. Further, polypeptides corresponding to subregions of the PRD sufficed to elicit the toxic effect on fast axonal transport, which was mediated by c-Jun N-terminal kinases (JNKs) and involved PRD binding to one or more SH3-domain containing proteins. Collectively, these data suggested a mechanism whereby polyQ tract expansion in mHTT promotes aberrant PRD exposure and interactions of this domain with SH3 domain-containing proteins including some involved in activation of JNKs. In support, biochemical and immunohistochemical experiments linked aberrant PRD exposure to increased JNK activation in striatal tissues of the zQ175 mouse model and from post-mortem Huntington's disease patients. Together, these findings support a critical role of PRD on mHTT toxicity, suggesting a novel framework for the potential development of therapies aimed to halt or reduce axonal pathology in Huntington's disease.
Keywords: Huntington’s disease; JNK3; SH3-binding domain; axonal transport; huntingtin; proline-rich domain.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests
Figures


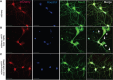


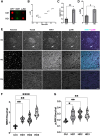

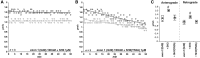
Comment in
-
Insights into the toxic effects of mutant huntingtin.Nat Rev Neurol. 2023 Oct;19(10):576. doi: 10.1038/s41582-023-00877-0. Nat Rev Neurol. 2023. PMID: 37697049 No abstract available.
References
-
- The Huntington's Disease Collaborative Research Group . A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993; 72:971–983. - PubMed
-
- Gusella JF, MacDonald ME. Molecular genetics: Unmasking polyglutamine triggers in neurodegenerative disease. Nat Rev Neurosci. 2000;1:109–115. - PubMed
-
- Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci. 2013;14:708–721. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

