Correlation of toxicities and efficacies of pemetrexed with clinical factors and single-nucleotide polymorphisms: a prospective observational study
- PMID: 37633908
- PMCID: PMC10464354
- DOI: 10.1186/s12885-023-11257-8
Correlation of toxicities and efficacies of pemetrexed with clinical factors and single-nucleotide polymorphisms: a prospective observational study
Abstract
Background: Pemetrexed is an efficacious multi-targeted antifolate with acceptable toxicity for non-squamous non-small cell lung cancer (non-Sq NSCLC) and malignant pleural mesothelioma. Vitamin B12 and folic acid as premedication can reduce the frequency of severe toxicities of pemetrexed chemotherapy. However, adverse effects are frequent in clinical settings. In this study, we aimed to identify the clinical factors and single-nucleotide polymorphisms (SNPs) associated with the toxicity and efficacy of pemetrexed chemotherapy.
Methods: This observational study was conducted from October 2012 to December 2019; we evaluated the toxicities and efficacies of pemetrexed chemotherapy using multivariate logistic or Cox regression analysis. In total, 106 patients received pemetrexed chemotherapy. SNPs were analyzed for four patients with malignant pleural mesothelioma and 67 with non-Sq NSCLC.
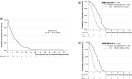
Results: The median progression-free survival (PFS) and overall survival of 63 patients with non-Sq NSCLC, excluding four in the adjuvant setting, were 6.8 and 33.3 months, respectively. Per propensity-score-adjusted multivariate Cox analyses, favorable factors for PFS were folic acid level ≥ 9.3 ng/mL before premedication, platinum combination, bevacizumab combination, vitamin B12 level < 1136 pg/mL before chemotherapy, A/A + A/G of BHMT (742 G > A), and A/A + A/C of DHFR (680 C > A). Favorable prognostic factors included good performance status, low smoking index, body mass index ≥ 20.66 kg/m2, folic acid level ≥ 5.55 ng/mL before premedication, higher retinol-binding protein before chemotherapy, and A/G of MTRR (66 A > G). Among the 71 patients who were analyzed for SNPs, the frequencies of hematologic toxicities and non-hematologic toxicities in Grades 3-4 were 38% and 36.6%, respectively. Per propensity-score-adjusted multivariate logistic analyses, risk factors for Grades 3-4 hematologic toxicities were vitamin B12 level < 486 pg/mL before premedication, leucocyte count < 6120 /µL before chemotherapy, folic acid level < 15.8 ng/mL before chemotherapy, status with a reduced dose of chemotherapy, and C/T + T/T of MTHFR (677 C > T). Risk factors for Grades 2-4 non-hematologic toxicities were homocysteine levels ≥ 11.8 nmol/mL before premedication, transthyretin level < 21.5 mg/dL before chemotherapy, C/C + T/T of MTHFR (677 C > T), and A/A + G/G of SLC19A1 [IVS2 (4935) G > A].
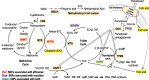
Conclusion: The information on metabolites and SNPs of the folate and methionine cycle will help predict the toxicities and efficacies of pemetrexed.
Trial registration: This trial was retrospectively registered with the University hospital Medical Information Network (UMIN000009366) on November 20, 2012.
Keywords: Betaine-homocysteine methyltransferase; Methionine cycle; Pemetrexed; Single-nucleotide polymorphism; Toxicities and efficacies.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
YT reports grants from Chugai and Boehringer Ingelheim and personal fees from MSD, Ono Pharmaceutical, and Otsuka Pharmaceutical Co. Ltd. outside the submitted work. GN reports personal fees from Astra Zeneca, Chugai Pharmaceutical, and Ono Pharmaceutical outside of the submitted work. KK reports personal fees (lecture fees) from MSD, Chugai, AstraZeneca, and Taiho Pharmaceutical Co. Ltd., outside the submitted work. YU reports personal fees from Chugai Pharmaceutical and Lilly outside of the submitted work. YK, MS, MH, and SH declare no conflicts of interest.
Figures




References
-
- National Comprehensive Cancer Network. NCCN Guidelines Version 3.2022, Non-Small Cell Lung Cancer. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 11 Aug 2022.
-
- National Comprehensive Cancer Network. NCCN Guidelines Version 1.2022, Malignant Pleural Mesothelioma. https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf. Accessed 11 Aug 2022.
-
- Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ, et al. Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther. 2002;1(7):545–552. - PubMed
-
- Kawazoe H, Yano A, Ishida Y, Takechi K, Katayama H, Ito R, et al. Non-steroidal anti-inflammatory drugs induce severe hematologic toxicities in lung cancer patients receiving pemetrexed plus carboplatin: a retrospective cohort study. PLoS One. 2017;12(2):e0171066. doi: 10.1371/journal.pone.0171066. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

