Practical diagnosis of cirrhosis in non-alcoholic fatty liver disease using currently available non-invasive fibrosis tests
- PMID: 37633932
- PMCID: PMC10460420
- DOI: 10.1038/s41467-023-40328-4
Practical diagnosis of cirrhosis in non-alcoholic fatty liver disease using currently available non-invasive fibrosis tests
Abstract
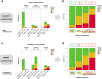
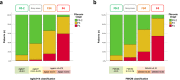
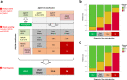
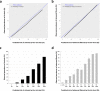
Unlike for advanced liver fibrosis, the practical rules for the early non-invasive diagnosis of cirrhosis in NAFLD remain not well defined. Here, we report the derivation and validation of a stepwise diagnostic algorithm in 1568 patients with NAFLD and liver biopsy coming from four independent cohorts. The study algorithm, using first the elastography-based tests Agile3+ and Agile4 and then the specialized blood tests FibroMeterV3G and CirrhoMeterV3G, provides stratification in four groups, the last of which is enriched in cirrhosis (71% prevalence in the validation set). A risk prediction chart is also derived to allow estimation of the individual probability of cirrhosis. The predicted risk shows excellent calibration in the validation set, and mean difference with perfect prediction is only -2.9%. These tools improve the personalized non-invasive diagnosis of cirrhosis in NAFLD.
© 2023. Springer Nature Limited.
Conflict of interest statement
Jerome Boursier: Board advisory and research grants to his institution from EchoSens. Other authors have nothing to declare.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

